Articles
- Page Path
- HOME > Acute Crit Care > Volume 36(2); 2021 > Article
-
Original Article
Pulmonary The effects of direct hemoperfusion with polymyxin B-immobilized fiber in patients with acute exacerbation of interstitial lung disease -
Jae Ha Lee1
 , Jin Han Park1
, Jin Han Park1 , Hyo-Jung Kim1
, Hyo-Jung Kim1 , Hyun Kuk Kim1
, Hyun Kuk Kim1 , Ji Hoon Jang1
, Ji Hoon Jang1 , Yong Kyun Kim2
, Yong Kyun Kim2 , Bong Soo Park3
, Bong Soo Park3 , Si Hyung Park3
, Si Hyung Park3 , Il Hwan Kim4
, Il Hwan Kim4 , Se Hun Kim5
, Se Hun Kim5 , Woon Heo6
, Woon Heo6 , Hang-Jea Jang1
, Hang-Jea Jang1
-
Acute and Critical Care 2021;36(2):126-132.
DOI: https://doi.org/10.4266/acc.2021.00073
Published online: April 15, 2021
1Division of Pulmonology, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
2Division of Infectious Diseases, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
3Division of Nephrology, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
4Division of Oncology, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
5Department of Anesthesiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
6Department of Thoracic and Cardiovascular Surgery, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- Corresponding author Hang-Jea Jang Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 48108, Korea Tel: +82-51-797-0100 Fax: +82-51-797-3009 E-mail: okabango21@gmail.com
- *These authors contributed equally to this work.
Copyright © 2021 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Acute exacerbation of interstitial lung disease (AE-ILD) causes clinically significant deterioration and has an extremely poor prognosis with high mortality. Recently, several studies reported the effectiveness of direct hemoperfusion with a polymyxin B-immobilized fiber column (PMX-DHP) in patients with AE-ILD as a potential therapy. This study describes the clinical effectiveness and safety of PMX-DHP in patients with AE-ILD.
-
Methods
- We retrospectively reviewed the medical records of 10 patients (11 episodes) with AE-ILD treated with PMX-DHP from January 2018 to June 2019. We compared laboratory and physiologic data of the ratio of partial pressure arterial oxygen to fraction of inspired oxygen (P/F ratio) and level of inflammatory markers before and after implementation of PMX-DHP.
-
Results
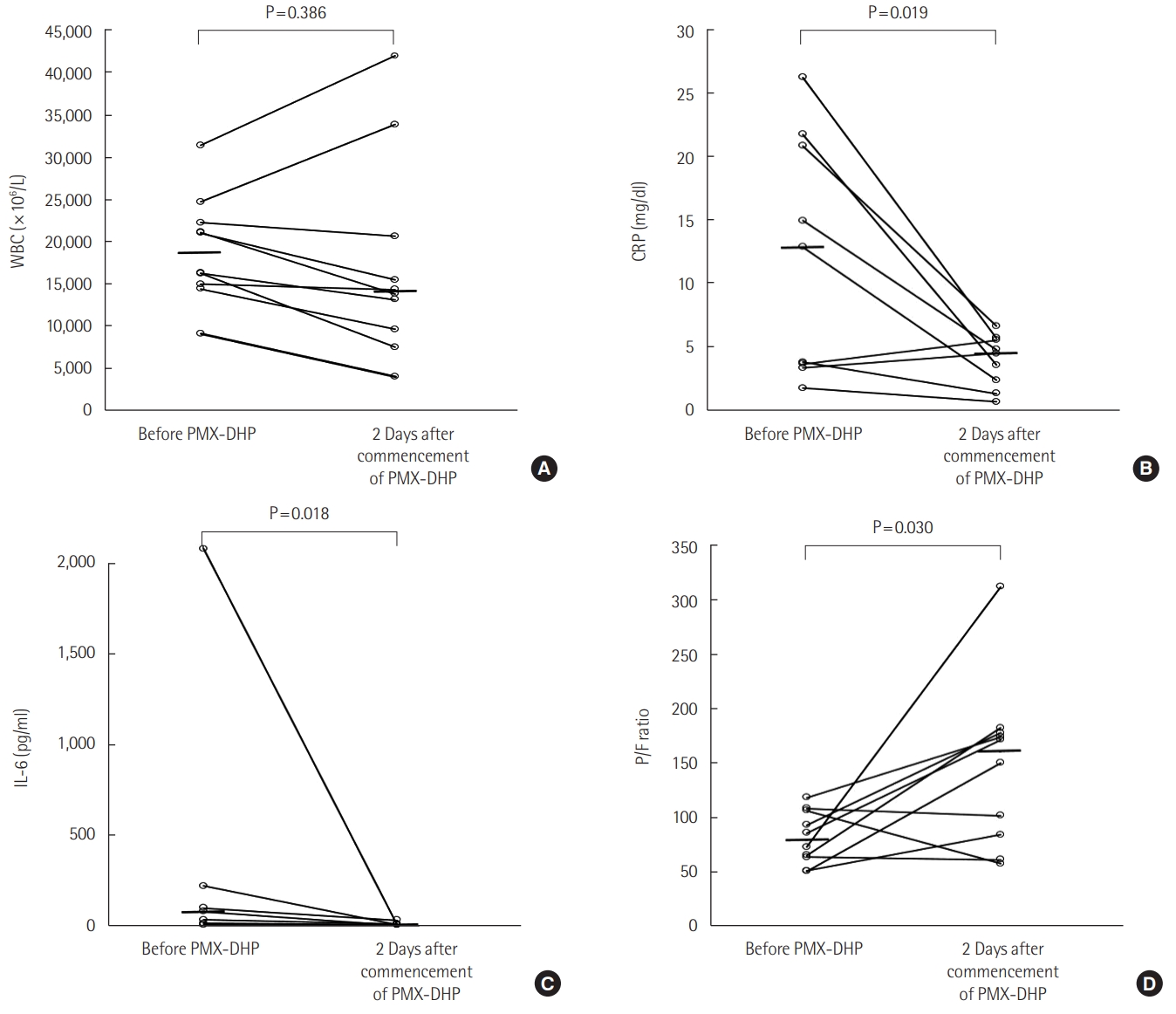
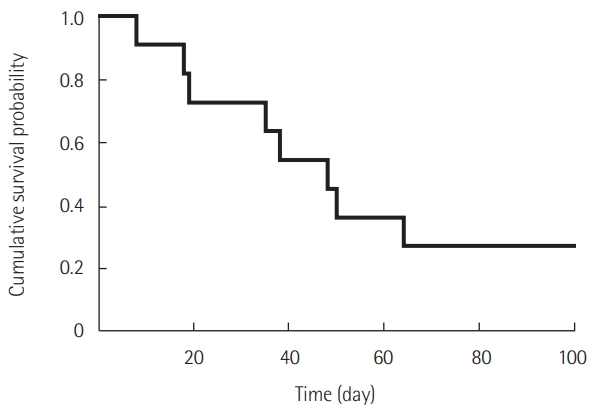
- Ten patients were included according to the 2016 revised definition of acute exacerbation of idiopathic pulmonary fibrosis (IPF). Nine patients had IPF and one patient had fibrotic nonspecific interstitial pneumonia. Most patients (90.9%) were treated with a steroid pulse, and four patients (36.4%) were treated with an immunosuppressant. The median number of PMX-DHP cycles was 2, and the median duration of each cycle was 6 hours. After PMX-DHP, the mean P/F ratio improved (86 [range, 63–106] vs. 145 [86–260], P=0.030) and interleukin-6 and c-reactive protein decreased (79 [35–640] vs. 10 [5–25], P=0.018 and 14 [4–21] vs. 5 [2–6], P=0.019, respectively). The 30-day mortality rate was 27.3% and the 90-day mortality rate was 72.7%.
-
Conclusions
- PMX-DHP treatment improved P/F ratio and reduced inflammatory markers in AE-ILD patients.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
KEY MESSAGES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conceptualization: JHL, HJJ. Data curation: JHL, JHP, JHJ, SHK, WH. Formal analysis & Methodology: JHL, JHP, HJK, HKK, YKK, IHK. Visualization: JHL, JHP, BSP, SHP. Writing–original draft: JHL, JHP. Writing–review & editing: all authors.
NOTES
| Characteristics | Value |
|---|---|
| Age (yr) | 66 (62–74) |
| Male sex | 7 (63.6) |
| BMI (kg/m2) | 24.1 (20.0–24.8) |
| Lung cancer | 3 (27.3) |
| Hypertension | 2 (18.2) |
| Diabetes mellitus | 1 (9.1) |
| Period from ILD diagnosis to AE (mo) | 13 (3–32) |
| GAP stage (I:II:III) | 2:6:3 |
| SOFA score | 5 (4–6) |
| Pulmonary function | |
| FVC, % predicted | 51 (47–66) |
| FVC, L measured | 2.1 (1.6–2.5) |
| DLCO, % predicted | 37 (35–46) |
| DLCO, L measured | 7.2 (5.7–8.3) |
| 6-Minute walk test | 11 (100.0) |
| Distance (m) | 345 (209–390) |
| Initial SpO2 (%) | 97 (90–98) |
| Lowest SpO2 (%) | 87 (76–93) |
| Initial P/F ratio | 86 (64–107) |
| PaO2 (mm Hg) | 64 (52–71) |
| PaCO2 (mm Hg) | 34 (33–40) |
| Serum WBC (×106/L) | 16,260 (14,400–22,210) |
| Serum CRP (mg/dl) | 14.9 (3.7–20.7) |
| Serum LDH (U/L) | 466 (428–582) |
| Serum lactate (mmol/L) | 2.0 (1.5–3.0) |
| Shocka | 6 (54.5) |
| Mechanical ventilation | 7 (63.6) |
| Steroid pulse therapy | 10 (90.9) |
| 500 mg methylPD per day | 5 (45.5) |
| 1 g methylPD per day | 5 (45.5) |
| Immunosuppressant | 4 (36.4) |
| Cyclosporin | 4 (36.4) |
| Cyclophosphamide | 2 (18.2) |
| ECMO | 4 (36.4) |
| 30-Day mortality | 3 (27.3) |
| 90-Day mortality | 8 (72.7) |
Values are presented as median (interquartile range) or number (%).
PMX-DHP: polymyxin B-immobilized fiber column; BMI: body mass index; ILD: interstitial lung disease; AE: acute exacerbation; GAP stage: genderage-physiology stage; SOFA: Sequential Organ Failure Assessment; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; SpO2: saturation of peripheral oxygen; P/F ratio: the ratio of partial pressure arterial oxygen to fraction of inspired oxygen; WBC: white blood cell; CRP: C-reactive protein; LDH: lactate dehydrogenase; methyl-PD: methylprednisolone; ECMO: extracorporeal membrane oxygenation.
a Shock: use of inotropic agent or vasopressor to maintain adequate tissue perfusion (mean arterial pressure greater than 65 mm Hg).
| Variable | Before 1st session | 48 Hours later | Crude P-value | Adjusted P-valuea |
|---|---|---|---|---|
| P/F ratio | 86 (63–106) | 145 (86–260) | 0.030 | 0.035 |
| IL-6 (pg/ml) | 79 (35–640) | 10 (5–25) | 0.018 | 0.408 |
| WBC (×106/L) | 16,260 (14,400–22,210) | 14,070 (9,132–23,917) | 0.386 | 0.608 |
| CRP (mg/dl) | 14 (4–21) | 5 (2–6) | 0.019 | 0.024 |
Values are presented as median (interquartile range).
P/F ratio: the ratio of partial pressure arterial oxygen to fraction of inspired oxygen; IL: interleukin; WBC: white blood cell; CRP: C-reactive protein; PMX-DHP: polymyxin B-immobilized fiber column.
a P-values were adjusted for application of mechanical ventilation, use of steroid pulse therapy, steroid dose, and use of immunosuppressant.
- 1. Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med (Lausanne) 2017;4:176. ArticlePubMedPMC
- 2. Usui Y, Kaga A, Sakai F, Shiono A, Komiyama K, Hagiwara K, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open 2013;3:e002971.ArticlePubMedPMC
- 3. Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63.ArticlePubMed
- 4. Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143-50.ArticlePubMed
- 5. Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J 2004;11:117-22.PubMed
- 6. Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial 2003;7:108-14.ArticlePubMed
- 7. Nakamura T, Kawagoe Y, Matsuda T, Shoji H, Ueda Y, Tamura N, et al. Effect of polymyxin B-immobilized fiber on blood metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 levels in acute respiratory distress syndrome patients. Blood Purif 2004;22:256-60.ArticlePubMed
- 8. Tsushima K, Kubo K, Koizumi T, Yamamoto H, Fujimoto K, Hora K, et al. Direct hemoperfusion using a polymyxin B immobilized column improves acute respiratory distress syndrome. J Clin Apher 2002;17:97-102.ArticlePubMed
- 9. Utsunomiya T, Mimura-Kimura Y, Yamamoto T, Aoe K, Oishi K, Kamei H, et al. Cytokine adsorption to polymyxin B-immobilized fiber: an in vitro Study. Blood Purif 2021;50:230-7.ArticlePubMed
- 10. Abe S, Azuma A, Mukae H, Ogura T, Taniguchi H, Bando M, et al. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med 2012;51:1487-91.ArticlePubMed
- 11. Hara S, Ishimoto H, Sakamoto N, Mukae H, Kakugawa T, Ishimatsu Y, et al. Direct hemoperfusion using immobilized polymyxin B in patients with rapidly progressive interstitial pneumonias: a retrospective study. Respiration 2011;81:107-17.ArticlePubMed
- 12. Seo Y, Abe S, Kurahara M, Okada D, Saito Y, Usuki J, et al. Beneficial effect of polymyxin B-immobilized fiber column (PMX) hemoperfusion treatment on acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2006;45:1033-8.ArticlePubMed
- 13. Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48.ArticlePubMedPMC
- 14. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824.ArticlePubMedPMC
- 15. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Respir Crit Care Med 2016;194:265-75.ArticlePubMed
- 16. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22.ArticlePubMed
- 17. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38.PubMed
- 18. Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428-46.ArticlePubMed
- 19. Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91.ArticlePubMed
- 20. Ueno T, Ikeda T, Ikeda K, Taniuchi H, Suda S, Yeung MY, et al. HMGB-1 as a useful prognostic biomarker in sepsis-induced organ failure in patients undergoing PMX-DHP. J Surg Res 2011;171:183-90.ArticlePubMed
- 21. Tani T, Hanasawa K, Kodama M, Imaizumi H, Yonekawa M, Saito M, et al. Correlation between plasma endotoxin, plasma cytokines, and plasminogen activator inhibitor-1 activities in septic patients. World J Surg 2001;25:660-8.ArticlePubMed
- 22. Oishi K, Mimura-Kimura Y, Miyasho T, Aoe K, Ogata Y, Katayama H, et al. Association between cytokine removal by polymyxin B hemoperfusion and improved pulmonary oxygenation in patients with acute exacerbation of idiopathic pulmonary fibrosis. Cytokine 2013;61:84-9.ArticlePubMed
- 23. Enomoto N, Mikamo M, Oyama Y, Kono M, Hashimoto D, Fujisawa T, et al. Treatment of acute exacerbation of idiopathic pulmonary fibrosis with direct hemoperfusion using a polymyxin B-immobilized fiber column improves survival. BMC Pulm Med 2015;15:15. ArticlePubMedPMC
- 24. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295.ArticlePubMedPMC
- 25. Papiris SA, Tomos IP, Karakatsani A, Spathis A, Korbila I, Analitis A, et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine 2018;102:168-72.ArticlePubMed
- 26. Kono M, Suda T, Enomoto N, Nakamura Y, Kaida Y, Hashimoto D, et al. Evaluation of different perfusion durations in direct hemoperfusion with polymyxin B-immobilized fiber column therapy for acute exacerbation of interstitial pneumonias. Blood Purif 2011;32:75-81.ArticlePubMed
- 27. Abe S, Seo Y, Hayashi H, Matsuda K, Usuki J, Azuma A, et al. Neutrophil adsorption by polymyxin B-immobilized fiber column for acute exacerbation in patients with interstitial pneumonia: a pilot study. Blood Purif 2010;29:321-6.ArticlePubMed
- 28. Takada T, Asakawa K, Sakagami T, Moriyama H, Kazama J, Suzuki E, et al. Effects of direct hemoperfusion with polymyxin B-immobilized fiber on rapidly progressive interstitial lung diseases. Intern Med 2014;53:1921-6.ArticlePubMed
- 29. Ichiyasu H, Horio Y, Masunaga A, Migiyama Y, Sakamoto Y, Jodai T, et al. Efficacy of direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) in rapidly progressive interstitial pneumonias: results of a historical control study and a review of previous studies. Ther Adv Respir Dis 2017;11:261-75.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Polymyxin B-immobilised fibre column treatment for acute exacerbation of idiopathic pulmonary fibrosis patients with mechanical ventilation: a nationwide observational study
Nobuyasu Awano, Taisuke Jo, Takehiro Izumo, Minoru Inomata, Yu Ito, Kojiro Morita, Hiroki Matsui, Kiyohide Fushimi, Hirokazu Urushiyama, Takahide Nagase, Hideo Yasunaga
Journal of Intensive Care.2023;[Epub] CrossRef - Changes in Oxygenation and Serological Markers in Acute Exacerbation of Interstitial Lung Disease Treated with Polymyxin B Hemoperfusion
Song-I Lee, Chaeuk Chung, Dongil Park, Da Hyun Kang, Jeong Eun Lee
Journal of Clinical Medicine.2022; 11(9): 2485. CrossRef

 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite



