Abstract
-
Background
- Extracorporeal membrane oxygenation (ECMO) is administered for a few days after lung transplantation (LTx) in recipients who are expected to have early graft dysfunction. Despite its life-saving potential, immediate postoperative ECMO has life-threatening complications such as postoperative bleeding. We investigated the risk factors related to the use of immediate postoperative ECMO.
-
Methods
- We retrospectively reviewed the records of 60 LTx patients who were at our institution from October 2012 to May 2015. Perioperative variables associated with postoperative ECMO were compared between the two groups.
-
Results
- There were 26 patients who received postoperative ECMO (ECMO group) and 34 patients who did not (control group). Multivariate regression analysis revealed preoperative ECMO (odds ratio [OR] 12.55, 95% confidence intervals [CI] 1.34 – 117.24, p = 0.027) and lower peripheral pulse oxymetry saturation (SpO2) at the end of surgery (OR 0.71, 95% CI 0.54 – 0.95, p = 0.019) were independent risk factors for postoperative ECMO in LTx patients. The incidences of complications, such as re-operation, tracheostomy, renal failure and postoperative atrial fibrillation, were higher in the ECMO group. There was no difference in the duration of postoperative intensive care unit stay or postoperative 30-day mortality between the two groups.
-
Conclusions
- The preoperative ECMO and lower SpO2 at the end of surgery were associated with postoperative ECMO. Further, postoperative adverse events were higher in the ECMO group compared with the control group. This study suggests that determination of postoperative ECMO requires careful consideration because of the risks of postoperative ECMO in LTx patients.
-
Keywords: extracorporeal membrane oxygenation; lung transplantation; perioperative; risk factor; weaning
Introduction
Over the past decades, lung transplantation has become an important therapeutic option for end-stage lung diseases.[1] In 1963, the first lung transplantation in humans was attempted by Dr. James Hardy at the University of Mississippi, and the recipient survived only 18 days. Since then, many advances have been made to improve outcomes and the International Society for Heart and Lung Transplantation reported that the median survival for all adult recipients was 7 years in bilateral lung recipients and 4.5 years in single lung recipients in the 2014 registry. While the survival rate has improved, the number of lung transplantations has grown steadily since 1985.[2]
Although several recipients are off-pump during lung transplantation, 30 to 40% of recipients are supported by intraoperative extracorporeal membrane oxygenation (ECMO) worldwide.[3,4] When the patient is expected to have a poor outcome like primary graft dysfunction (PGD) if intraoperative ECMO is stopped, the surgeon determines whether to use postoperative ECMO support at the end of surgery, as a bridge to recovery of the transplanted lungs. However, despite its life-saving potential, ECMO requires high-dose anticoagulation and can be associated with life-threatening complications like hemorrhage, renal failure, thromboembolism, cannulation-related injury and sepsis.[5-8] For these reasons, it is important that postoperative ECMO is only selectively used.
Until now, although there have been many studies about the risk factors of PGD,[4,7-10] there is a lack of studies about which preoperative factors and intraoperative situations influence the surgeon’s decision to use postoperative ECMO. Therefore the aim of the present study was to evaluate perioperative factors associated with the use of postoperative ECMO in the immediate postoperative period. Additionally, we investigated the difference in clinical outcomes comparing the postoperative ECMO group and intraoperative ECMO weaning group.
Materials and Methods
1) Study protocol
After obtaining the approval from the Institutional Review Board of Severance Hospital, we retrospectively investigated all patients undergoing lung transplantation from October 2012 to May 2015 in Severance Hospital. We included patients who had undergone one or two lung transplantations with intraoperative ECMO support by one surgeon. We excluded patients who underwent lung transplantation without intraoperative ECMO support, or combined with another surgery like coronary artery bypass surgery. Patients who had incomplete data associated with perioperative ECMO were also excluded. The patients were divided into two groups. The control group consisted of patients who were weaned from intraoperative ECMO at the end of surgery. The ECMO group consisted of patients for whom the intraoperative ECMO was prolonged to the immediate postoperative period for a few days, so that the transplanted lungs could recover.
2) Data collection
We collected perioperative data from electronic medical records of eligible patients. The preoperative variables, including patient’s demographics, functional status, laboratory finding, past medical history, use of preoperative ECMO, waiting days to operation and diagnosis for lung transplantation, were investigated. All preoperative variables were based on data from within the last 6 months before lung transplantation. Intraoperative variables included duration of anesthesia and operation, ECMO time, transfusion, blood loss, laboratory values, peak airway pressure and hemodynamics. Postoperative variables included the number of reoperations, complications (including re-intubation, tracheostomy, renal failure, stroke, atrial fibrillation, gastrointestinal bleeding and stress induced cardiomyopathy), length of stays in hospital or intensive care unit (ICU) and postoperative 30 days mortality. Donor’s values also were obtained from the records of the organ transplantation department in our hospital. The data from the donors included age, gender, the ratio of arterial oxygen partial pressure to the fraction of inspired oxygen concentration (PaO2/FiO2) and cold ischemic time.
3) Perioperative ECMO management
During the operation, veno-arterial ECMO (VA ECMO) was used in all patients. Before cannulation, patients received 3,000 to 5,000 IU of intravenous unfractionated heparin. After confirming an activated clotting time (ACT) greater than 200 seconds, central or peripheral cannulation for ECMO was performed. The arterial cannulation sites were the aorta or femoral artery, and the venous cannulation site was the right atrium or femoral vein. ECMO flow started at 20 mL/kg/min (1-2 L/min) and slowly increased over a few minutes until sufficient flow was achieved. The ECMO flow was maintained at 60 to 80 mL/kg/min (3.5-5.0 L/min) with 2 to 6 L/min sweep gas flow at 100% oxygen. To achieve a target ACT of 180 to 200 seconds, ACT was measured every 30 minutes and additional heparin was administered. After implanted lung anastomosis and reperfusion, the duration of ECMO weaning was evaluated by the lung transplantation team. The ECMO flow was decreased by 500 to 1,000 mL/min for consideration of ECMO weaning. Finally, the decision to wean or maintain the intraoperative ECMO depended on the attending surgeon. When the use of postoperative ECMO was determined, most patients went from intraoperative VA ECMO to veno-venous ECMO (VV ECMO); however, some patients with hemodynamic instability combined with pulmonary hypertension or right heart failure were kept on VA ECMO and transferred to the ICU.
4) Statistical analysis
SPSS for Windows (version 20.0, SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Then, the variables showing normality were analyzed using the Student’s t-test and expressed as the mean ± standard deviation. The variables not showing normality were analyzed using the Mann-Whitney test and expressed as the median (25 interquartile-75 interquartile). Multiple logistic regression analysis using backwards stepwise regression was performed. Variables with a level of significance defined as p < 0.20 for univariate logistic regression analysis, as well as clinically important variables, were entered as candidate variables in the multivariate models to assess their viability as an independent predictor for the postoperative ECMO. The results are reported as odds ratios (OR) with 95% confidence intervals (CI) and the relevant p-value. To assess the predictive power of the logistic regression model, a receiver operating characteristic (ROC) curve was used and we calculated the area under the curve (AUC). Generally, we consider a p-value < 0.05 as significant, but due to the small size of this study, this was insufficient to identify a difference. Therefore, based on previous reports, we considered a p-value < 0.1 to be significant.[11]
Results
Between October 2012 and May 2015, there were 64 patients who had undergone lung transplantation. Among these, 4 patients were excluded; 2 patients had undergone combined coronary artery bypass surgery, one patient had undergone simultaneous lung and liver transplantation and the other patient had insufficient data due to unknown preoperative ECMO insertion day and management. A total of 60 patients were enrolled in our study. Among the eligible patients, 34 were weaned from intraoperative ECMO (Control group) and 26 patients were included in the postoperative ECMO (ECMO group).
Patient’s preoperative characteristics and comparison between the control group and the ECMO group are described in Table 1. Aside from the use of preoperative ECMO, all other preoperative variables, including demographics, functional status, past medical history and diagnosis for lung transplantation, were not significantly different. The use of preoperative ECMO was lower in the Control group than in the ECMO group (Control group: 3% vs. ECMO group: 23%, p = 0.036). The donors’ characteristics are described in Table 2. There were no significant variables among donors’ characteristics.
Comparison of intraoperative variables between the control group and ECMO group are described in Table 3. Most patients (88%) underwent double lung transplantation, while 7 patients (12%) underwent single lung transplantation due to a size mismatch between donor and recipient, bad lung condition of donor or bad intraoperative condition of recipient. The operation and anesthesia times were not significantly different between the two groups. One aspect of fluid input and output, namely the amount of total fluid, was more in the ECMO group than in the Control group (control group: 7,205 ± 2,755 mL vs. ECMO group: 8,927 ± 4,521 mL, p = 0.075). Further, there were more units of transfused packed red blood cell in the ECMO group than in the control group (Control group: 5 units[3-9] vs. ECMO group: 8 units [5-14], p = 0.029). However, blood loss did not differ between the groups, and the amount of bleeding was a little higher in the control group compared to the ECMO group (Control group: 2,175 mL [1,425-3,225] vs. ECMO group: 2,115 mL [1,300-7,725], p = 0.164). When the determination was made for intraoperative ECMO weaning or maintenance, the peripheral pulse oxymetry saturation (SpO2) (Control group: 100 [100-100] vs. ECMO group: 100 (95-100), p = 0.014) and peak inspiratory pressure (Control group: 21 cmH2O vs. ECMO group: 23 cmH2O, p = 0.002) were significantly higher in the ECMO group compared to the control group. In contrast, pulmonary artery pressure (PAP) was not significantly different.
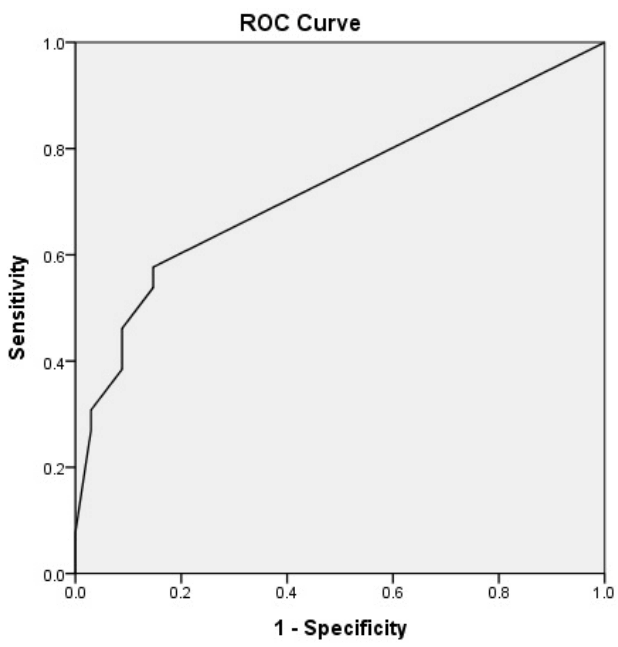
In consideration of the multicollinearity between multiple variables, the number of patients who used preoperative ECMO, donor PaO2/FiO2 and SpO2 at the end of surgery were included in the multiple logistic regression models to identify an independent predictor for the use of postoperative ECMO, as shown in Table 4. The use of preoperative ECMO (OR 12.55, 95% CI 1.34–117.24, p = 0.027) and low SpO2 (OR 0.71, 95% CI 0.54–0.95, p = 0.019) at the moment of determination for intraoperative ECMO weaning were found to independently predict the use of postoperative ECMO, respectively. To assess the predictive power of the logistic regression model, a ROC curve was used and the AUC was 0.727 (95% CI 0.59–0.86, p = 0.003) (Fig. 1).
Analysis of postoperative outcomes and complications is described in Table 5. Although the length of ICU stay was not different, the length of hospital day was longer in the ECMO group than in the Control group (Control group: 53 ± 46 days vs. ECMO group: 73 ± 42 days, p = 0.077). Further, postoperative 1-day blood loss was higher in the ECMO group than in the Control group (Control group: 1,563 ± 1,188 mL vs. ECMO group: 2,920 ± 2,288 mL, p = 0.004). The mean number of postoperative mechanical ventilator days was 5 in the ECMO group and 1 in the Control group (p = 0.008). The postoperative complications occurred more often in the ECMO group than in the Control group. In particular, re-operation, tracheostomy, and renal failure showed significantly lower incidences in the Control group. There was no difference in postoperative 30-day mortality (p = 1.000).
Discussion
This study was conducted with the aim of assessing perioperative factors in predicting the use of postoperative ECMO. In multivariable analysis, we found that the use of preoperative ECMO and low SpO2 at the end of surgery was associated with the use of postoperative ECMO.
At the end of surgery, the decision regarding whether to wean or maintain intraoperative ECMO depended on the attending surgeon due to the absence of optimal parameters. If the intraoperative ECMO was stopped, the surgeon sometimes chose postoperative ECMO support when hemodynamic instability or poor outcomes like graft dysfunction were expected. In our experience of 2 years and 8 months, 43% (26/60) of patients were transferred from the operation room to the ICU with ECMO support. This percentage is a little higher than that reported in recent studies by Ius et al[3] (41% [19/46]) and Aigner et al[4] (39% [51/130]).
Although a definite indication for the use of postoperative ECMO after lung transplantation has not yet been widely investigated, the most common indication for the use of postoperative ECMO at ICU is related to PGD.[4,7-9,12-14] PGD is defined by the presence of hypoxemia and radiographic infiltrates, assessed at time points up to 72 hours (within 6 hours of reperfusion, 24, 48, and 72 hours).[15] The incidence of PGD has been reported to vary from 5 to 30% of patients.[16,17] PGD is associated with increased mechanical ventilation, longer stays in ICU, and increased probability of bronchiolitis obliterans syndrome. Also, PGD is the most important cause of early mortality and morbidity after lung transplantation.[12,14] Therefore, many centers have studied the parameters for occurrence of PGD after lung transplantation.[7,16]
In the many studies over the use of postoperative ECMO at ICU, only a few reports have referred to the postoperative ECMO transfer from the operation room. In one report by Aigner et al[4], the factors influencing the decision for postoperative ECMO support included aspects of the donor, recipient and intraoperative situation. They indicated that a high-risk recipient has elevated PAP, marginal donor organ quality and progressively deteriorating graft function with a decreasing oxygenation index, especially, combined with pulmonary hypertension associated with the use of postoperative ECMO.[4]
In our multiple logistic regression model, significant variables included the use of preoperative ECMO (OR 12.55) and SpO2 (OR 0.71) at the end of intraoperative ECMO (Table 4). In the ROC curve analysis of factors in the multiple logistic model, the AUC was 0.727. These results show that the model has the power to predict the use of postoperative ECMO. Unlike in the previous literature described by Aigner et al[4], we could not found a difference in preoperative or intraoperative PAP and donor organ quality between the two groups. We suggested that the surgeon was actually influenced by preoperative patient disease severity and oxygen index right after reperfusion.
Like other invasive procedures, postoperative ECMO management has the potential for complications.[5-8,18] The common complications include bleeding, renal failure, infection, stroke, arrhythmia and limb ischemia. In particular, the major complication was bleeding because ECMO needs heparin infusion to maintain an activated clotting time of 180 to 250 seconds.[18] In our study, wound drainage of postoperative 1 day in the ECMO group (2,920 ± 2,288 mL) was more than that in the Control group (1,563 ± 1,188 mL) (Table 5). The incidence of bleeding that required reoperation was 35% (9/26) in the ECMO group versus 6% (2/34) in the Control group. Other complications aside from stroke occurred more in the ECMO group than in the control group, although some variables were not significantly different. These complications lead to prolonged mechanical ventilation and increased length of ICU stays. However, postoperative 30-day mortality showed no significant difference between the groups, despite the lower complications in the Control group. Based on previous reports that early initiation of ECMO for postoperative PGD leads to more optimal outcomes than delayed initiation of ECMO,[10,13,18] we assumed that the use of postoperative ECMO had a prophylactic effect for postoperative PGD, resulting in no significant difference in survival. The main weakness of this study was the paucity of eligible patients, so further work is needed to evaluate the adverse effects of postoperative ECMO on the long-term clinical outcomes such as 5-year survival rate.
When considering postoperative ECMO support after lung transplantation, VV ECMO was preferable to VA ECMO because VA ECMO had more risk of complications than VV ECMO.[19] In this study, postoperative VA ECMO was applied to only 4 patients among the 26 patients of ECMO group. The incidence of complications was higher in the VA ECMO group than the VV ECMO group, although only the differences in atrial fibrillation (100% in VA ECMO group vs. 22.7% in VV ECMO group, p = 0.008) and stroke (50% in VA ECMO group vs. 0% in VV ECMO group, p = 0.018) were statistically significant. The expire rate was also significantly higher in the VA ECMO group than the VV ECMO group (75% vs. 22.7%, respectively, p = 0.072).
There were several limitations to our study. First, this study was performed at a single center and the sample size was relatively small, so the study was underpowered for several variables. Second, because this was a retrospective analysis, data collection and interpretation of data were limited. Each patient had different time intervals from last preoperative clinical evaluation to operation day and the general condition of the recipients deteriorated rapidly, although we collected preoperative clinical findings within 6 months from the operation day. Thus, we could not know the precise status of the patient’s clinical functional before lung transplantation. Also, we could not retrospectively obtain details about inotropic drug dose, transesophageal echocardiography findings and ECMO flow at timing of intraoperative ECMO weaning. Third, there were several potential confounders such as differences in technical skills of the surgeon and assistants over time. Fourth, because the data from recent patients were included, mortality was investigated until postoperative 30 day. Therefore, it is possible that a significant difference in mortality could not be detected.
In summary, we investigated factors associated with postoperative ECMO in the immediate postoperative period after lung transplantation. The preoperative use of ECMO and intraoperative low SpO2 at the end of surgery were factors related to the use of postoperative ECMO. Further, the postoperative ECMO support was associated with more postoperative complications such as prolonged duration of mechanical ventilation, prolonged hospital stays, bleeding that required re-operation, tracheostomy, renal failure that required dialysis and atrial fibrillation, although there was no significant difference in postoperative 30-day mortality. We suggest further exploration through a large prospective study to evaluate additional factors and the effect on mortality to construct a predictive model for the use of postoperative ECMO after lung transplantation.
NOTES
-
No potential conflict of interest relevant to this article was reported.
Fig. 1.Receiver operating characteristic (ROC) curve for multiple regression model. The area under the receiver operating characteristic curve was 0.727 (95% CI 0.59-0.86, p = 0.003).

Table 1.Preoperative patient’s characteristics for control and ECMO groups before lung transplantation
|
Control (n = 34) |
ECMO (n = 26) |
p-value |
|
Age, yr |
49 ± 16 |
51 ± 12 |
0.448 |
|
Male |
22 (65) |
17 (65) |
1.000 |
|
Weight, kg |
55 ± 10 |
54 ± 9 |
0.982 |
|
FEV1, % |
41 ± 21 |
45 ± 20 |
0.401 |
|
FVC, % |
44 ± 16 |
39 ± 16 |
0.272 |
|
FEV1/FVC, % |
72 ± 5 |
82 ± 19 |
0.129 |
|
Left ventricular ejection fraction, % |
62 ± 11 |
64 ± 8 |
0.376 |
|
Right ventricular systolic pressure, mmHg |
52 ± 20 |
55 ± 29 |
0.693 |
|
Arterial blood gas analysis |
|
|
|
|
pH |
7.4 ± 0.1 |
7.4 ± 0.1 |
0.912 |
|
PaCO2
|
45 ± 19 |
42 ± 19 |
0.564 |
|
PaO2
|
59 ± 15 |
59 ± 22 |
0.914 |
|
HCO3-
|
28 ± 8 |
26 ± 7 |
0.316 |
|
6-minute walk, m |
270 (123 - 327) |
210 (120 - 285) |
0.161 |
|
Waiting time, day |
74 (34 - 199) |
52 (20 - 125) |
0.399 |
|
Mechanical ventilation |
7 (21) |
9 (35) |
0.252 |
|
ECMO |
1 (3) |
6 (23) |
0.036*
|
|
Smoking |
16 (47) |
13 (50) |
1.000 |
|
Past medical history |
|
|
|
|
Chronic obstructive pulmonary disease |
2 (6) |
3 (12) |
0.644 |
|
Asthma |
2 (6) |
1 (4) |
1.000 |
|
Chronic renal failure |
3 (9) |
1 (4) |
0.626 |
|
Hypertension |
8 (24) |
4 (15) |
0.526 |
|
Diabetes mellitus |
8 (24) |
5 (19) |
0.760 |
|
Pulmonary tuberculosis |
6 (18) |
2 (8) |
0.446 |
|
Coronary artery disease |
4 (12) |
1 (4) |
0.377 |
|
Stroke |
0 (0) |
2 (8) |
0.184 |
|
Hepatitis |
4(12) |
1(4) |
0.377 |
|
Pneumonia |
7(20) |
7(27) |
0.759 |
|
Diagnosis for lung transplantation |
|
|
|
|
Idiopathic pulmonary fibrosis |
19 (32) |
14 (23) |
0.875 |
|
Bronchiolitis obliterans organizing pneumonia |
7 (12) |
4 (7) |
0.742 |
|
Bronchiectasis |
2 (3) |
1 (2) |
1.000 |
|
Autoimmune related interstitial lung disease |
3 (5) |
6 (10) |
0.157 |
|
Lymphangioleiomyomatosis |
3 (5) |
0 (0) |
0.251 |
|
Idiopathic pulmonary hypertension |
0 (0) |
1 (2) |
0.433 |
Table 2.Donor’s variables for control and ECMO groups
|
Control (n = 34) |
ECMO (n = 26) |
p-value |
|
Ages, yr |
40 ± 10 |
45 ± 13 |
0.178 |
|
Male, n (%) |
20 (59) |
17 (65) |
0.789 |
|
PaO2/FiO2 ratio |
442 ± 115 |
404 ± 102 |
0.187 |
|
Ischemic time, min |
238 ± 60 |
272 ± 97 |
0.108 |
Table 3.Intraoperative variables for control and ECMO groups during lung transplantation
|
Control (n = 34) |
ECMO (n = 26) |
p-value |
|
Operation time, hr |
6.8 ± 1.6 |
7.2 ± 1.6 |
0.211 |
|
Anesthesia time, hr |
8.4 ± 1.7 |
9.0 ± 1.8 |
0.372 |
|
Transplanted lung number (unilateral:bilateral) |
(3 : 31) |
(4 : 22) |
0.454 |
|
Total fluid, mL |
7,205 ± 2,755 |
8,927 ± 4,521 |
0.075*
|
|
Total colloid, mL |
1,000 (500-1000) |
100 (500-1500) |
0.989 |
|
Transfusion, unit |
|
|
0.685 |
|
Packed red blood cell |
5 (3-9) |
8 (5-14) |
0.029*
|
|
Fresh frozen plasma |
3 (1-5) |
3 (0-5) |
0.939 |
|
Platelet |
6 (0-12) |
6 (0-12) |
0.648 |
|
Blood loss, mL |
2,175 (1425-3225) |
2,115 (1300-7725) |
0.164 |
|
At the end of intraoperative ECMO |
|
|
|
|
SpO2, % |
100 (100-100) |
100 (95-100) |
0.014*
|
|
Mean pulmonary artery pressure, mmHg |
23 ± 7 |
26 ± 9 |
0.264 |
|
Cardiac output, L/min |
3.3 (3.0-4.0) |
3.5 (2.0-5.0) |
0.537 |
|
Peak inspiratory pressure, cmH2O |
21 (16-23) |
23 (20-29) |
0.006*
|
Table 4.Multivariable-adjusted OR and 95% CI of factors influencing failure to wean from intraoperative ECMO
|
Characteristics |
Univariable analysis
|
Multivariable analysis
|
|
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
|
Preoperative use of ECMO |
9.90 (1.11-88.34) |
0.040*
|
12.55 (1.34–117.24) |
0.027*
|
|
Donor PaO2/FiO2
|
1.00 (0.99-1.00) |
0.193 |
1.00 (0.99–1.00) |
0.352 |
|
Total fluid input during operation |
1.00 (1.00–1.00) |
0.147 |
|
|
|
Transfused pRBC during operation |
1.09 (1.00-1.19) |
0.087*
|
|
|
|
SpO2 at the end of surgery |
0.74 (0.57-0.97) |
0.028*
|
0.71 (0.54–0.95) |
0.019*
|
|
PIP at the end of surgery |
1.21 (1.05-1.40) |
0.007*
|
|
|
Table 5.Postoperative variables for control and ECMO groups after lung transplantation
|
Control (n = 34) |
ECMO (n = 26) |
p-value |
|
Length of hospital stays, day |
53 ± 46 |
73 ± 42 |
0.077*
|
|
Length of intensive care unit stays, day |
21 ± 2 |
33 ± 37 |
0.172 |
|
Postoperative 1 day wound drainage, mL |
1,563 ± 1,188 |
2,920 ± 2,288 |
0.004*
|
|
Postoperative ventilator day, day |
5 (3-39) |
19 (9-73) |
0.008*
|
|
30-day mortality |
5 (16) |
4 (16) |
1.000 |
|
Re-operation |
7 (21) |
14 (54) |
0.013*
|
|
Re-intubation |
9 (27) |
10 (53) |
0.405 |
|
Tracheostomy |
9 (27) |
14 (54) |
0.037*
|
|
Renal failure required dialysis |
5 (15) |
10 (39) |
0.069*
|
|
Stroke/CVA |
4 (12) |
2 (8) |
0.689 |
|
Atrial fibrillation |
4 (12) |
9 (35) |
0.056*
|
|
Gastrointestinal bleeding |
3 (9) |
4 (15) |
0.454 |
|
Stress induced cardiomyopathy |
3 (9) |
3 (12) |
0.528 |
References
- 1. Hardy JD, Webb WR, Dalton ML Jr, Walker GR Jr. Lung homotransplantation in man. JAMA 1963;186:1065-74.ArticlePubMed
- 2. Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24.ArticlePubMed
- 3. Ius F, Kuehn C, Tudorache I, Sommer W, Avsar M, Boethig D, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6.ArticlePubMed
- 4. Aigner C, Wisser W, Taghavi S, Lang G, Jaksch P, Czyzewski D, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg 2007;31:468-73.ArticlePubMed
- 5. Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 2013;15:172-8.ArticlePubMed
- 6. Lee YJ, Kim DJ, Kim JS, Lee JH, Lee CT, Jheon S, et al. Experience and results with VV-ECMO for severe acute respiratory failure: weaning versus nonweaning. ASAIO J 2015;61:184-9.ArticlePubMed
- 7. Fischer S, Bohn D, Rycus P, Pierre AF, de Perrot M, Waddell TK, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO) registry. J Heart Lung Transplant 2007;26:472-7.ArticlePubMed
- 8. Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis 2014;6:1070-9.PubMedPMC
- 9. Glassman LR, Keenan RJ, Fabrizio MC, Sonett JR, Bierman MI, Pham SM, et al. Extracorporeal membrane oxygenation as an adjunct treatment for primary graft failure in adult lung transplant recipients. J Thorac Cardiovasc Surg 1995;110:723-6.ArticlePubMed
- 10. Hartwig MG, Walczak R, Lin SS, Davis RD. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg 2012;93:366-71.ArticlePubMed
- 11. Lentner C. Geigy Scientific Tables, Vol. 2: Introduction to Statistics Statistical Tables Mathematical Formulae. 8th ed. Basle, Switzerland, Ciba-Geigy. 1982, pp 156. -62. 163.
- 12. Bermudez CA, Adusumilli PS, McCurry KR, Zaldonis D, Crespo MM, Pilewski JM, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg 2009;87:854-60.ArticlePubMed
- 13. Castleberry AW, Hartwig MG, Whitson BA. Extracorporeal membrane oxygenation post lung transplantation. Curr Opin Organ Transplant 2013;18:524-30.ArticlePubMed
- 14. Oto T, Rosenfeldt F, Rowland M, Pick A, Rabinov M, Preovolos A, et al. Extracorporeal membrane oxygenation after lung transplantation: evolving technique improves outcomes. Ann Thorac Surg 2004;78:1230-5.ArticlePubMed
- 15. Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D, ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454-9.ArticlePubMed
- 16. Nosotti M, Palleschi A, Rosso L, Tosi D, Mendogni P, Righi I, et al. Clinical risk factors for primary graft dysfunction in a low-volume lung transplantation center. Transplant Proc 2014;46:2329-33.ArticlePubMed
- 17. Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg 2013;145:1065-70.ArticlePubMed
- 18. Meyers BF, Sundt TM, Henry S, Trulock EP, Guthrie T, Cooper JD, et al. Selective use of extracorporeal membrane oxygenation is warranted after lung transplantation. J Thorac Cardiovasc Surg 2000;120:20-6.ArticlePubMed
- 19. Hartwig MG, Appel JZ 3rd, Cantu E 3rd, Simsir S, Lin SS, Hsieh CC, et al. Improved results treating lung allograft failure with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2005;80:1872-80.ArticlePubMed
Citations
Citations to this article as recorded by

- The Future of Research on Extracorporeal Membrane Oxygenation (ECMO)
Ji Young Lee
Korean Journal of Critical Care Medicine.2016; 31(2): 73. CrossRef
 , Sungwon Na, M.D., Ph.D.1
, Sungwon Na, M.D., Ph.D.1 , Hyo Chae Paik, M.D., Ph.D.2, Jonglin Ha, M.D.3, Jeongmin Kim, M.D., Ph.D.1
, Hyo Chae Paik, M.D., Ph.D.2, Jonglin Ha, M.D.3, Jeongmin Kim, M.D., Ph.D.1






 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite