Successful percutaneous coronary intervention with extracorporeal membrane oxygenation support after right coronary artery dissection in an eisenmenger syndrome patient
Article information
Abstract
The presentation of coronary artery disease in a patient with Eisenmenger syndrome (ES) is relatively rare. Cardiogenic shock due to coronary artery dissection during percutaneous coronary intervention (PCI) can be more critical in these patients. Here, we report a case of successful PCI under mechanical circulation support in a patient with ES who experienced potentially fatal right coronary artery dissection. This case emphasizes that use of extracorporeal membrane oxygenation (ECMO) can lead to successful management of critical complication during PCI, and that the immediate decision to apply of ECMO is important in ES patients who face impending cardiogenic shock with acute heart failure.
Eisenmenger syndrome (ES) occurs as a consequence of a large congenital left to right cardiac shunt that leads to pulmonary hypertension and subsequently a cyanotic right to left shunt. Percutaneous coronary intervention (PCI) in ES patients can be a high risk procedure especially when it provokes unpredictable complications that lead to cardiogenic shock and acute heart failure. Immediate extracorporeal membrane oxygenation (ECMO) insertion should be considered when critical complications occur in ES patients during PCI.
CASE REPORT
A 52-year-old male was admitted to Seoul Paik Hospital due to aggravated dyspnea and exertional chest pain that had developed a week ago. He was diagnosed with ES due to an unrepaired large atrial septal defect (ASD) at 8 years prior and had been taking sildenafil and macitentan (endothelin receptor antagonist) for pulmonary hypertension. Except for smoking, he had no other traditional risk factors for atherosclerosis. Electrocardiography showed right ventricular (RV) hypertrophy with strain pattern (Figure 1A). Chest X-ray showed cardiomegaly and an enlarged pulmonary trunk. Transthoracic echocardiography revealed RV hypertrophy and enlargement compressing the left ventricle, marginally depressed RV function, dilated pulmonary arteries, markedly elevated estimated RV systolic pressure of about 158 mmHg, which was derived from tricuspid regurgitation velocity, and a large ASD secundum (2.37 cm) (Figure 1B). Routine examination revealed blood pressure of 130/80 mmHg, heart rate of 92/min, and peripheral oxygen saturation of 89% at room air. Laboratory tests revealed hemoglobin of 16.7 g/dL, white blood cell of 6,220 cells/mm3, platelet of 161×103/mm3, blood urea nitrogen of 16 mg/dL, creatinine of 1.03 mg/dL, and pro-brain natriuretic peptide of 210.1 pg/mL.
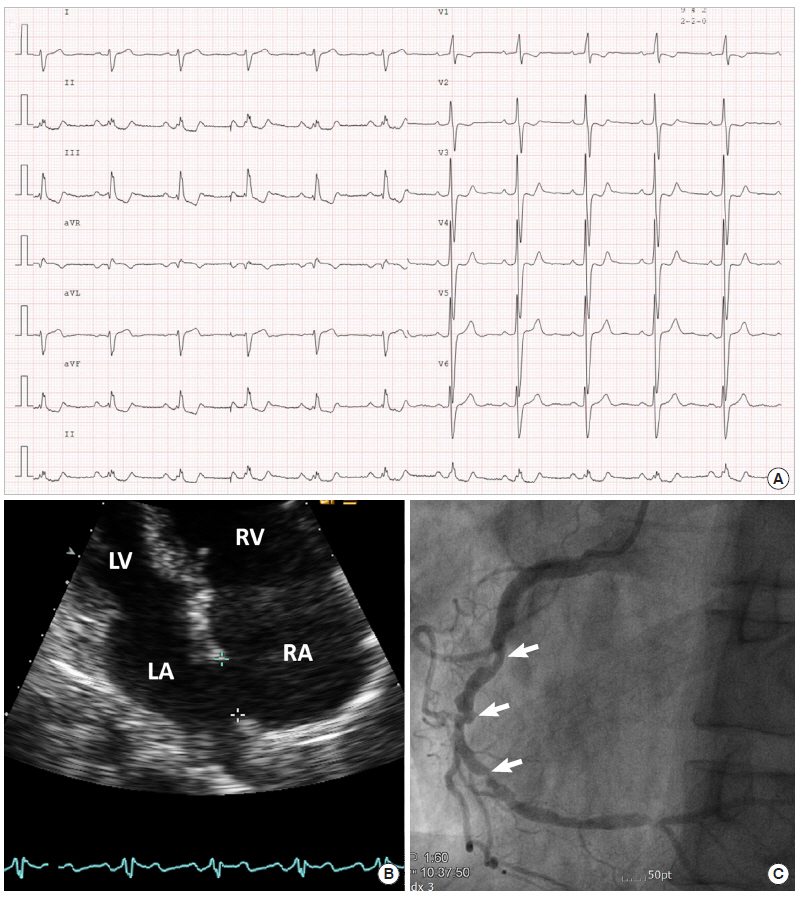
Baseline examination. (A) Electrocardiography showing sinus rhythm and right ventricular hypertrophy. (B) Echocardiography. Apical four chamber view showing right atrial and ventricular dilatation and atrial septal defect. (C) Coronary angiography. Angiographic image showing significant luminal narrowing in the mid to distal right coronary artery. LV: left ventricle; RV: right ventricle; LA: left atrium; RA: right atrium.
Coronary angiography via the right radial artery indicated a nonocclusive left coronary artery, but found significant stenosis of the mid to distal right coronary artery (RCA) (Figure 1C). With use of 8,000 units of heparin, we tried to engage the 6-F Amplatz left guiding catheter into RCA, but the catheter was engaged deeply. In the cine after engagement, we found dissection of the proximal RCA with a collapsed lumen and diminished coronary flow (Thrombolysis In Myocardial Infarction [TIMI] grade 0 flow) (Figure 2). Inferior ST-segments were elevated on electrocardiography and the patient complained of ongoing chest pain with agitation. His blood pressure was dropped to 72/51 mmHg at first and marginally maintained after rapid administration of 600 ml crystalloids and initiation of inotropes (dopamine 15 μg/min/kg and norepinephrine 0.1 μg/min/kg). We decided to insert a veno-arterial extracorporeal membrane oxygenation (ECMO) immediately prior to PCI. A 17-F arterial and a 21-F venous Bio-Medicus cannula (Medtronic, Minneapolis, MN, USA) were percutaneously inserted into the right femoral vessels and venous cannula was positioned at right atrium under fluoroscopic guidance. A PLS system circuit (Maquet, Rastatt, Germany) and ROTAFLOW console (Maquet) were used. After initiation of ECMO, his blood pressure was recovered to 100/60 mmHg. We changed the guiding catheter to a 6-F Judkins right 4.0 catheter and passed the guidewire successfully through the true lumen (Figure 3A). We performed direct stenting with two drug eluting stents (each 3.5×18 mm; Resolute Onyx, Medtronic) at the proximal section and ostium of the RCA to cover the dissection, and coronary blood flow was restored (TIMI grade 3 flow) (Figure 3B and C). A significant lesion of the mid to distal RCA was treated with another drug eluting stent (3.0×38 mm; Resolute Onyx, Medtronic) (Figure 3D). Final angiography revealed no residual stenosis or dissection. The patient was monitored carefully in the intensive care unit after PCI. We initially started with an ECMO flow rate of 4.0 L/min, considering patient body weight. We monitored arterial blood gas analysis via both right radial artery and ECMO arterial cannula. Difference of PaO2 between upper and lower extremities (right radial artery: PaO2, 68 mmHg; saturation, 95% vs. ECMO arterial cannula: PaO2, 426.5 mmHg; saturation, 100%) was noted, which means left ventricle systolic function was sufficient to supply right carotid artery against retrograde flow from ECMO. However, he kept complaining of dizziness and his blood pressure decreased to 82/66 mmHg when a high ECMO flow rate was applied. The bedside echocardiography revealed that left ventricular systolic function was preserved, but preload seemed to be insufficient. The hemoglobin level decreased from 16.7 g/dL to 12.2 g/dL after ECMO apply. Two pints of packed red blood cells were transfused and the hemoglobin level recovered to 14.2 g/dL. Fluid resuscitation with crystalloids via both intravenous route and ECMO circuit was also effective to raise the patient’s blood pressure. With volume replacement, we reduced the ECMO flow rapidly and maintained the flow as low as possible (approximately 1.5 L/min). Intravenous heparin was infused during ECMO support with targeted activated partial thromboplastin time of 60–80 seconds and activated clotting time of 200–220 seconds. His vital signs remained stable for 7 hours despite of minimal ECMO support. ECMO was successfully removed using a percutaneous closure device (Perclose; Abbott Vascular, Abbott Park, IL, USA) without any vascular complications. The patient was discharged with improved clinical symptoms and was stable for 3 months of follow-up in the outpatient clinic.
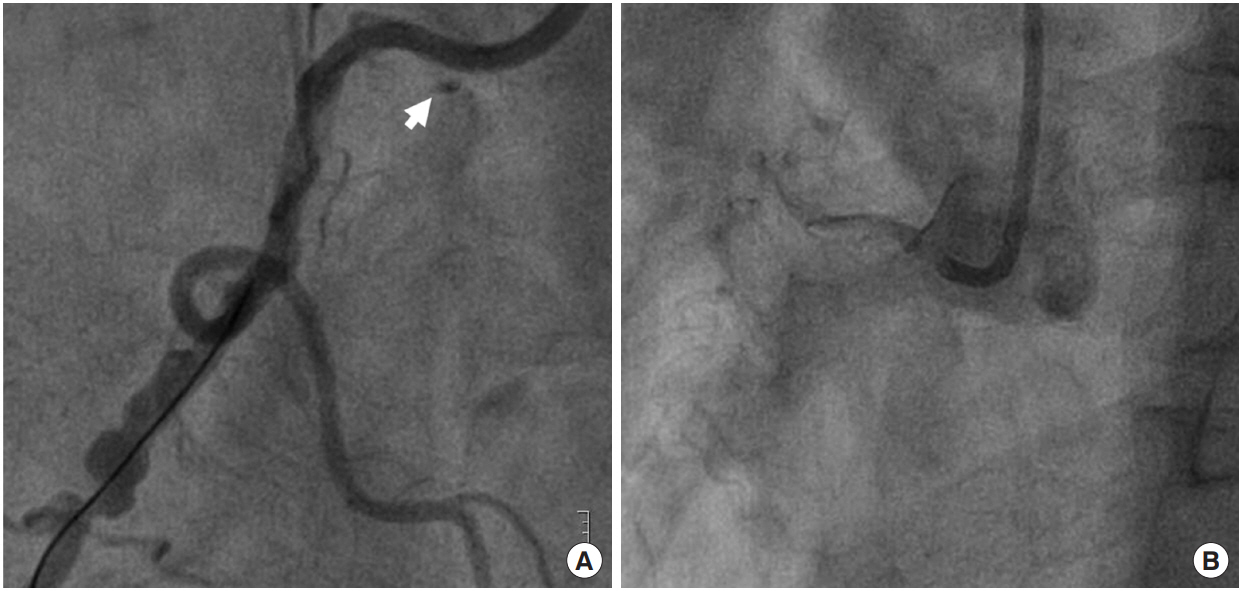
Angiography after guiding catheter engagement. (A) The cine showing angiographic haziness at the proximal right coronary artery and contrast stain suggesting dissection at the ostium (arrow). (B) Collapsed and invisible coronary vessel lumen due to dissection.
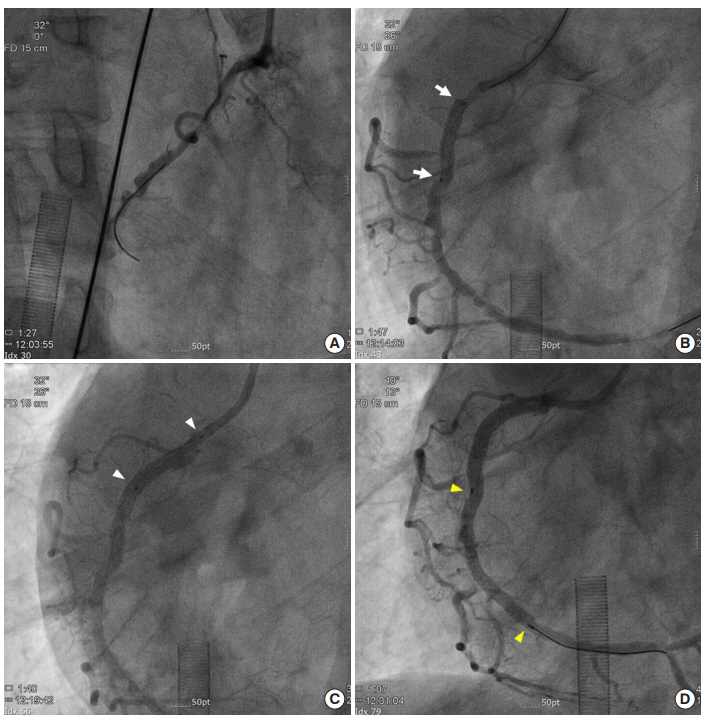
Direct percutaneous coronary intervention under extracorporeal membrane oxygenation support. (A) Successful wiring through the true lumen. (B) Direct stenting at the proximal to mid right coronary artery (RCA) to cover the dissection (white arrows, Onyx 3.5×18 mm). (C) Direct stenting at the RCA ostium (white arrowheads, Onyx 3.5×18 mm). (D) Direct stent deployment at the mid to distal RCA (yellow arrowheads, Onyx 3.0×38 mm).
DISCUSSION
Concomitant coronary atherosclerosis in patients with cyanotic congenial heart disease is rarely reported. The possible reasons related to the low incidence of ischemic heart disease in this group include a relatively short life expectancy, ectatic change of coronary arteries owing to endothelial shear stress induced by erythrocytosis with subsequent release of endothelial vasodilator substances [1], and combined thrombocytopenia or hypocholesterolemia that might have an antiatherogenic effect [2].
Since the introduction of advanced pulmonary vasodilator therapy, however, median survival of Eisenmenger patients has increased and now reaches up to 52.3 years [3]. In addition, systemic endothelial dysfunction had been described in cyanotic heart disease, which can be a further deteriorating factor in myocardial ischemia [4]. Our case demonstrates that patients with ES do not have resistance to the development of obstructive coronary artery disease as their survival improves. Therefore, physicians should pay attention to conventional risk factor modification in Eisenmenger patients age 50 years or older.
The major cause of death in patients with ES is heart failure [3]. In particular, acute decompensated heart failure can be fatal in these patients. However, there is no exact guidelines when we should use ECMO in patients with ES. In this case, the timing of ECMO insertion was relatively early at impending cardiogenic shock, not overt shock state. Although, intraaortic balloon pump (IABP) could be an alternative option in this case, we considered the patient’s specific condition underlying ES with higher RV loading state unlike the patients without structural heart disease. We concerned the refractory cardiogenic shock due to RV infarction and failure as a result of RCA dissection with total collapse in this case. Furthermore, we could not assure immediate successful recanalization of the true lumen of dissected coronary artery. We thought that only augmentation of coronary perfusion supported by IABP may not be sufficient and veno-arterial ECMO might allow attenuation of RV pressure overload and rescue from RV failure by bypassing the resistant pulmonary circulation. Therefore, we promptly inserted ECMO prior to PCI and successfully removed it.
However, the following concerns should be considered in the management of ECMO in ES patients. First, ES patients are sensitive to intravascular volume status. Adequate volume replacement and transfusion might be important when ECMO was started since acute volume loss of right atrium occurred by venous cannula and hemodilution by the priming volume. In addition, a prolonged high flow rate of ECMO can reduce the left ventricular preload in patients with ES. According to this case, we propose that a lower ECMO flow rate might be better in patients with ES compared to general recommendation. Bleeding complications are another concern during ECMO support in ES patients after PCI. During maintenance of a low ECMO flow rate, intensive anticoagulation may be necessary to reduce the chance of thrombosis. Use of dual antiplatelet and anticoagulation treatment in ES patients is a concern since these patients frequently suffer from thrombocytopenia and coagulopathy. The proper flow rate of ECMO and target range for anticoagulation in ES patients remain unknown because there are no guidelines and few published cases with successful management of ECMO in such patients [5,6]. Further experience is needed to address these issues and establish the exact role and optimal safe parameters for ECMO in ES patients with acute and critical heart failure.
We present a successful PCI case with ECMO support in a patient with ES who nearly faced cardiogenic shock and acute RV failure due to RCA dissection. This case highlights the usefulness and safety of ECMO and the importance of an immediate decision to apply ECMO in patients with ES when serious complications occur during PCI.
Notes
No potential conflict of interest relevant to this article was reported.
