Rapid response systems in Korea
Article information
Abstract
The inpatient treatment process is becoming more and more complicated with advanced treatments, aging of the patient population, and multiple comorbidities. During the process, patients often experience unexpected deterioration, about half of which might be preventable. Early identification of patient deterioration and the proper response are priorities in most healthcare facilities. A rapid response system (RRS) is a safety net to identify antecedents of these adverse events and to respond in a timely manner. The RRS has become an essential part of the medical system worldwide, supported by all major quality improvement organizations. An RRS consists of a trigger system and response team and needs constant assessment and process improvement. Although the effectiveness and cost-benefit of RRS remain controversial, according to previous studies, it may be beneficial by decreasing in-hospital cardiac arrest and mortality. Since the first implementation of RRS in Korea in 2008, it has been developed in over 15 medical centers and continues to expand. Recent accreditation standards and an RRS pilot program by the Korean government will promote the proliferation of RRSs in Korea.
INTRODUCTION
It is well known that approximately 10% of patients admitted to hospital experience unexpected serious adverse events [1]. A rapid response system (RRS) is a patient safety strategy that prevents cardiac arrests or deaths by providing immediate and timely interventions when patients unexpectedly deteriorate [2,3]. Delayed or inappropriate medical management in these patients may result in an increased risk of death or disability. An RRS aims to improve the safety of hospital-ward patients. Although the effectiveness of RRSs is controversial [4,5], several before-and-after studies have shown a reduction in cardiac arrest and hospital mortality [6-11]. Starting in the United States and Australia, this system has become an essential part of patient safety and has been adopted worldwide. Last year, the Korea Institute for Healthcare Accreditation distributed its third edition revised accreditation standards, and it recommended all acute care hospitals implement an RRS. In addition, in May 2019, the Korean Health Insurance Review and Assessment Service and the Ministry of Health and Welfare started a rapid response system pilot program. This article reviews the concept of the RRS; its requirements; and its past, present, and future in Korea.
RAPID RESPONSE SYSTEM
Patient Safety Issues
Admitted patients experience unexpected adverse events in about 10% of cases, 7.3% of which could be fatal. Although not all unexpected adverse events are preventable or predictable, 30% to 83% are so [1,12,13]. Approximately 80% of in-hospital cardiorespiratory arrest showed at least one abnormal sign such as blood pressure, heart rate, respiratory rate, body temperature, or change in consciousness within 8 hours before the event [14-16]. This suggests that there is an opportunity for intervention before patient condition worsens. However, these antecedents can go unrecognized in general wards. In most hospitals, continuous monitoring of vital signs is usually available only in the intensive care unit (ICU). The number of physician rounds for ward-patients is one to two times a day. The interval between measurements of vital signs is usually 8 hours or longer. Respiratory rate and mental state should be measured under direct observation, which is prone to error [15,17]. Even if there is a warning sign, it may not be recognized or properly alerted depending on personal experience, attitude, work environment, and position of the responding nurse or the doctor. As there is a long chain of command to activate—from nurse to intern or resident, from resident to fellow, fellow to attending physician—an alert may be delayed in each step [2].
In the surgical ward, doctors are not readily available because they might be in the operating room. Even if the patient’s warning signs are recognized and reported in a timely manner, it can be difficult to provide appropriate monitoring, intervention, or treatment because of the limited medical resources available in general wards. The Surviving Sepsis Campaign emphasizes early recognition and treatment. Accordingly, the current guideline combines 3- and 6-hour bundles into a “1-hour bundle” to emphasize immediate resuscitation and management [18]. Considering this, the current conventional medical system might be inadequate for proper early response.
Components of RRS
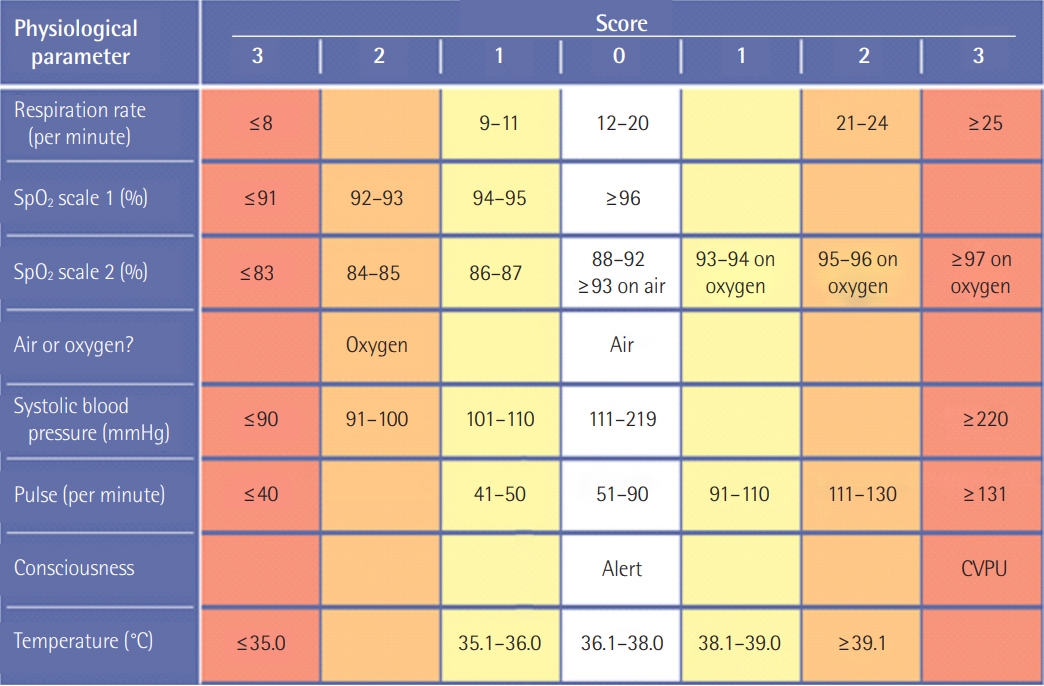
An RRS is composed of doctors and nurses who specialize in intensive care medicine to cope with patients at risk. An RRS consists of an afferent limb, efferent limb, patient safety, quality improvement with feedback from data analysis, and administrative components including education of staff [3], as shown in Figure 1. The afferent limb is a way to detect patient deterioration [19]. A nurse or a ward-physician can contact the responding team according to “calling criteria.” Alternatively, using scores such as the modified Early Warning Score (MEWS), patients at risk can be actively screened [20,21]. As under-recognition of monitored abnormal values leads to delay in activation, a surveillance system using a scoring system like MEWS (Table 1) [20] or the National Early Warning Score (NEWS) (Figure 2) [22] can be useful for detecting unexpected adverse events.
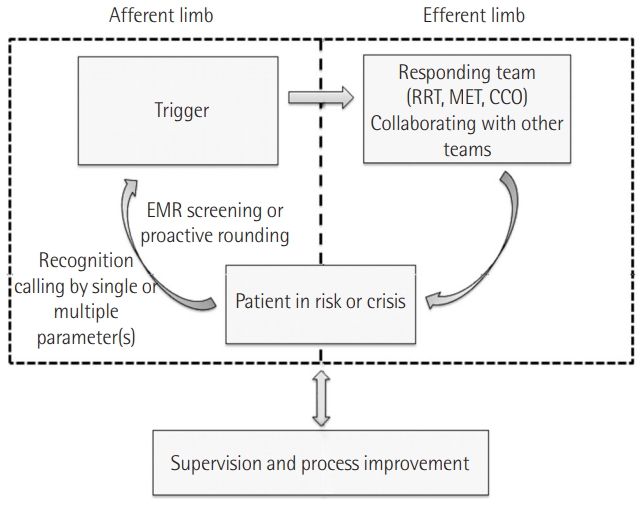
Structure of the rapid response system. RRT: rapid response team; MET: medical emergency team; CCO: critical care outreach; EMR: electronic medical recording.
Once deterioration has been detected, the responding team works as an efferent limb. The forms may vary—for example, doctor and nurse can work as a team, or the nurse can respond first, and the doctor can cooperate when necessary. According to the composition and character of the responding team, it can be referred to as a rapid response team (RRT), medical emergency team (MET), critical care outreach (CCO) program, or critical care transit program. An RRT is often a nurse-led team. An MET is usually physician-led, offering several critical care interventions such as airway management, central vascular line insertion, and resuscitation. A CCO program adds active surveillance for patients at risk to RRT, managing high-risk patients in advance. The responding team should be equipped with real-time monitoring devices, vascular access devices, oxygen delivery devices, and intervention medications [3].
Continuous quality improvement including self-assessment and data analysis with feedback is another important part in RRS. This quality improvement limb is essential to prevent or prepare for future events. To determine event rates, resource requirements and outcomes, indicators such as number of calls, reason for call, unit where the call started, time of each call, number of transfers to ICU, and the number of calls converted to cardiopulmonary resuscitation (CPR) are collected. Monthly meeting for review of potentially avoidable cases would be an effective strategy for quality improvement.
Implementation of RRS in Other Countries
The Institute for Healthcare Improvement started the “100,000 Lives Campaign” in 2004 to engage U.S. hospitals to prevent 100,000 needless inpatient deaths. To reach this goal, the Institute for Healthcare Improvement suggested six clinical interventions including deployment of an RRT. About 3,100 hospitals (about 3/4 of all hospitals in the United States) were enrolled in the campaign and prevented an estimated 122,300 needless deaths [23]. Sixty percent of hospitals enrolled in the campaign implemented an RRS to detect and intervene in those in crises. As a result, cardiac arrest rate in the general ward was reduced by 50%, transfer from ward to ICU decreased by 58%, and overall mortality decreased by 37%. The Institute for Healthcare Improvement followed with the 5 Million Lives Campaign, and related campaigns were launched in Canada, Australia, Sweden, Denmark, and the UK.
This system is widely supported by accreditors and quality improvement organizations such as the Joint Commission in the USA. The 2009 Joint Commission’s National Patient Safety Goal recommended implementation of an RRS [24]. The 2015 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care also recommended it to reduce the incidence of cardiac arrest, particularly in the general care ward [25]. As a result, an RRS is now implemented in more than 3,700 hospitals throughout the United States and has spread to other continents including Europe and Asia [2,4,26-28].
The preferred composition of the responding team differs among countries and institutions. In the UK, the RRS team may be nurse-led [29], and in the USA, nurses or respiratory therapists may lead [27]. In Australia, New Zealand, and Scandinavia, a physician-led RRS is favored [26,30].
Effectiveness of RRS
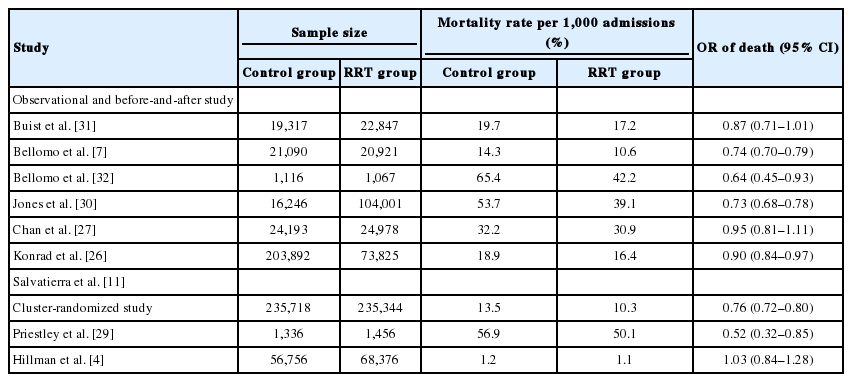
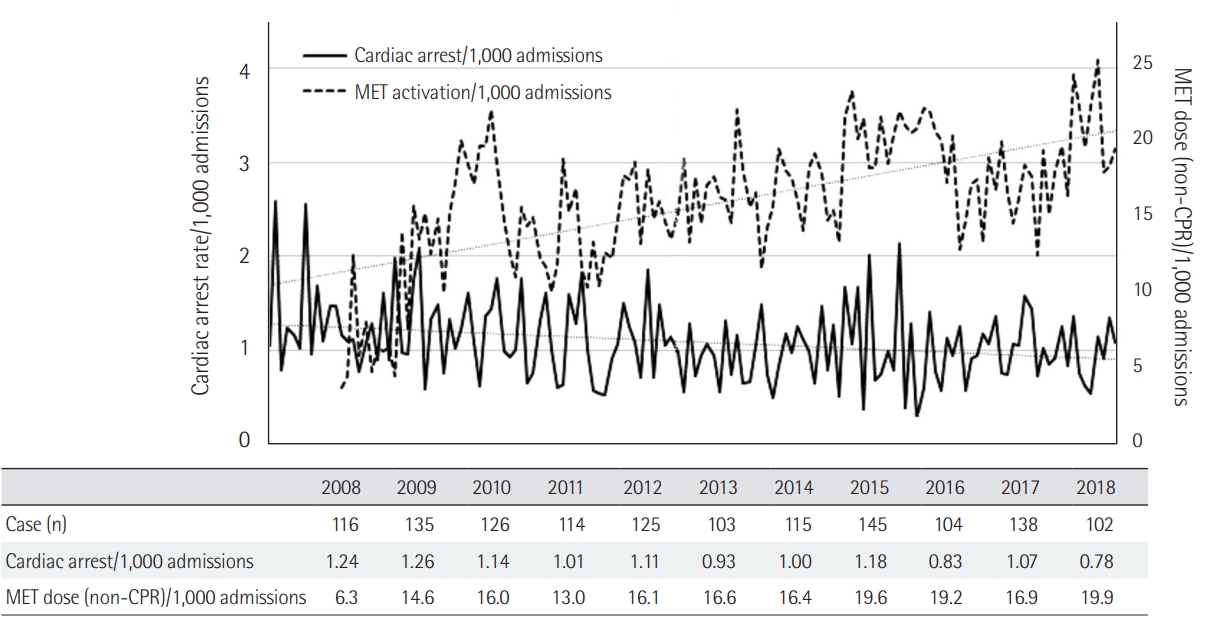
The Medical Early Response Intervention and Therapy (MERIT) trial [4] was a multicenter, cluster-randomized controlled trial of MET, which failed to demonstrate benefit regarding composite endpoint of death, unexpected cardiac arrest, and unplanned ICU admission. However, in the MERIT trial, the cardiac arrest response team was utilized in the control arm about 50% of the time, even before the actual event of cardiac arrest. This may have decreased the gap between the control and the intervention arm with RRS. In the past, the effects of RRS were controversial, but in recent years, there have been solid results supporting this system. Many observational before-and-after studies showed that RRS resulted in decreased in-hospital mortality and cardiac arrest [6-11,30]. The study by Salvatierra et al. [11] was an observational cohort study including 471,062 adult patients from 2001 to 2009. It revealed that RRS improved in-hospital mortality by 24% compared to the pre-RRT time period. Another recent retrospective study found a significant decrease in unexpected and overall mortality [10]. Table 2 is a summary of studies reporting in-hospital mortality [4,7,11,26,27,29-32]. Although the studies mentioned above show considerable heterogeneity, a meta-analysis by Maharaj et al. [33] has consistently shown similar effectiveness of RRS. Implementation of RRS in the adult population was associated with a reduction of overall hospital mortality and cardiorespiratory arrest. In a tertiary hospital in Korea (Asan Medical Center), we found that the number of inhospital cardiac arrests decreased with increase of RRS calls (Figure 3). Based on these results, implementation of RRSs and annual RRT calls are increasing [34].
Past and Present of RRS in Korea
Despite the lack of accurate data related to patient safety events in the Korean medical system, one study found that about 7% of patients experienced at least one adverse event, and that 61% of events were preventable. In Korea, use of an RRS started in 2008, and the number of hospitals operating an RRS has since been increasing. Asan Medical Center first started an RRS in March 2008; this response team was called the medical alert team (MAT). At first, this system was applied to limited patients as a pilot project. It then was expanded to every patient in the hospital in May 2009. In addition to a calling system by attending physician or nurse in the general ward, the MAT in Asan Medical Center is based on a surveillance program using an electronic medical recording (EMR) screening system [35]. Since MAT was implemented, several hospitals started to prepare for RRS. Samsung Medical Center also prepared an RRS and started pilot operation in January 2009, followed by Hanyang University Hospital in 2011 [36], Seoul National University Bundang Hospital in 2012 [37], and Seoul St. Mary’s Hospital in 2013. Since then, awareness of RRS has been heightened due to promotions in several critical care-related conferences and academic meetings.
To implement RRS successfully, several barriers should be overcome, especially the culture. Korean culture is characterized by a somewhat shy and hierarchical relationship between doctors and nurses. Thus, the amount of direct calling for RRS is small at the beginning. Many Korean RRS overcame low calling rate by introducing EMR screening method including vital signs, labs, and proactive rounds (Figure 4).
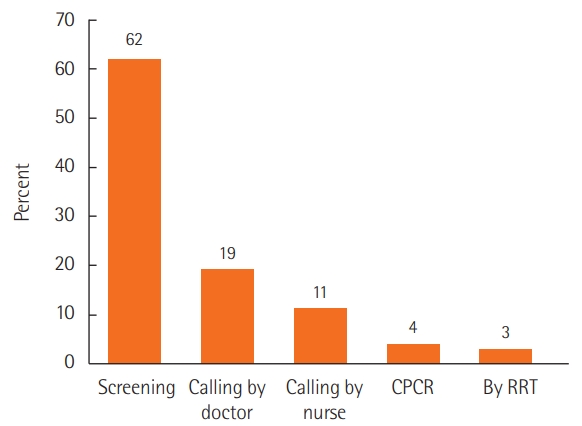
Types of activation of rapid response system from Korean multicenter data (n=11,646). CPCR: cardiopulmonary cerebral resuscitation; RRT: rapid response team.
Korean RRS was the topic of several papers during this period [35-42]. A single-center before-and after-study of RRS by Kwak et al. [36] and a Korean multicenter study [39] showed that RRS decreased in-hospital cardiac arrest. Kim et al. [38] compared in-hospital cardiac arrest rates by RRS working hours and showed that the incidence of in-hospital cardiac arrest was decreased only during RRS operating hours. RRS not only decreased in-hospital cardiac arrest rates, but also improved mortality from sepsis and first-attempt success rate of intubation (Figures 5, 6) [43,44]. However, many hospitals do not operate 24 hours a day, and physicians dedicated to such a team are rare. Most medical staff are working as intensivists and as members of the RRS team at the same time and are vulnerable to mental and physical exhaustion.
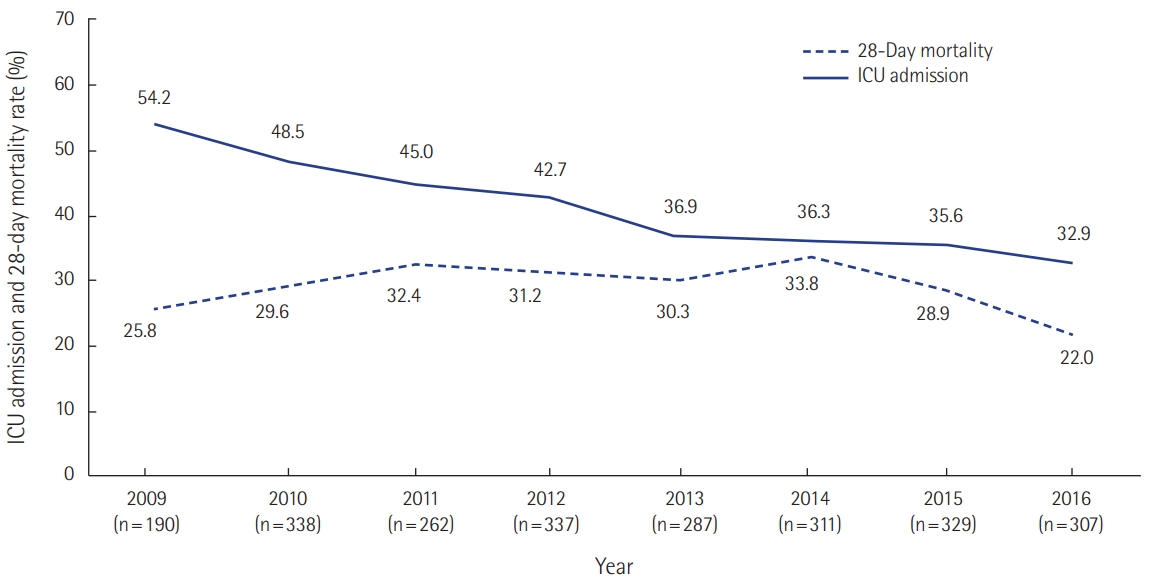
Outcomes of rapid response system activation in sepsis (n=2,361). ICU: intensive care unit. Unpublished data from Asan Medical Center.
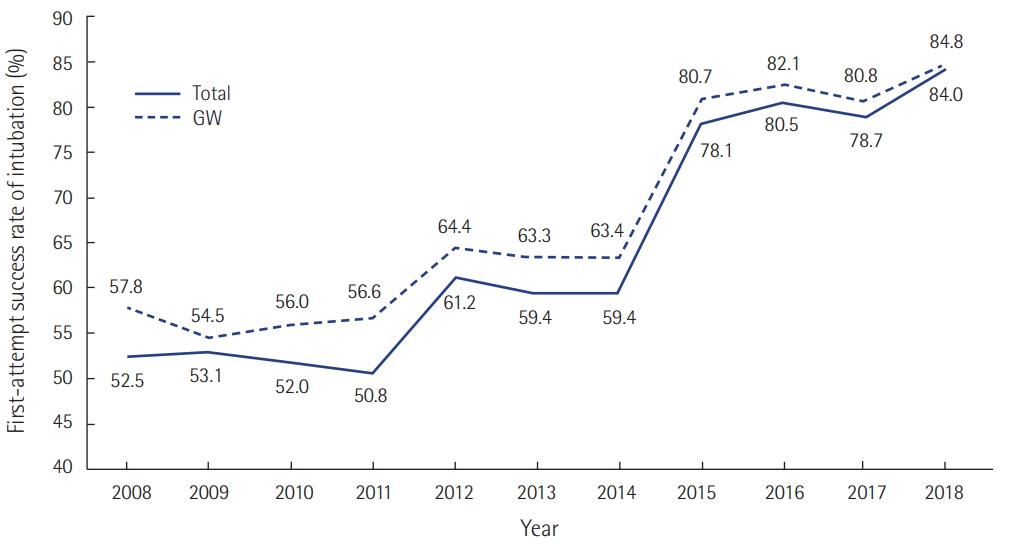
The first-attempt success rate of intubation has improved with the successful implementation of a rapid response system, in the general ward (GW) and the entire hospital. A responding team in rapid response system included experienced operator for airway management. Unpublished data from Asan Medical Center.
Leaders in hospitals know that an RRS is a good system. There is particular value in Korea where there are not enough medical personnel and resources. However, implementation of an RRS is potentially expensive, and there was no RRS management fee until now. Thus, investment in RRS has been challenging. In 2018, as the Korea Institute for Healthcare Accreditation mandated items for patient safety improvement, more hospitals are adopting an RRS.
2019 is a milestone in the history of RRS in Korea. In May 2019, the Korean Health Insurance Review and Assessment Service and Ministry of Health and Welfare started an RRS pilot program, expanding RRSs further. The third edition revised accreditation standards for acute care hospital accreditation included implementation of an RRS. These changes are expected to make RRS an essential part of improving patient safety. Through introduction of the RRS, hospital mortality rate, number of cardiac arrests, length of ICU stay, and rate of readmission to ICU may be reduced. This might lead to a reduction in overall medical costs by improving the quality of medical care.
Future of RRS in Korea
In 2019, more than 50 hospitals in Korea will run an RRS. Many hospitals are just beginning to overcome obstacles in implementing an RRS such as lack of awareness of the system, activation criteria, response protocol, organizational culture, resource scarcity, and fear of appearing inappropriate [45]. Constant, repeated education, feedback for quality improvement, and administrative support should be used to move forward. The optimal composition of RRS is not certain. It should be decided according to available institutional resources, preferences, and goals. Simulation training for RRS responders and hospital staffs can result in better management and communication. Appropriate calling and screening criteria as an afferent limb are essential. A novel informatics approach might be an option in the near future [46]. As wearable devices are more widely used, data from these may become available, leading to more accurate and timely assessment. As more hospitals adopt the RRS, associated Korean multicenter data will accumulate and will enable future development.
CONCLUSIONS
Since its introduction in the 2000s, the RRS has spread all over the world. Several studies have shown that this system is effective in reducing hospital mortality and in-hospital cardiac arrest. As it is becoming an essential part of the medical system for improving patient safety, clinicians need to understand the history and rationale of RRS and its potential benefits, limitations, and strategies for successful implementation. Although the history of RRS in Korea is not long, we have made great strides this year with administrative effort and support. With continued effort, we hope the RRS in Korea will evolve further in the future.
KEY MESSAGES
▪A rapid response system (RRS) is a patient safety system that prevents adverse events by providing immediate interventions when patients unexpectedly deteriorate.
▪Several studies have shown that implementation of an RRS results in reduction of unexpected mortality and cardiac arrest.
▪In Korea, more than 15 hospitals have implemented an RRS, and this is expected to increase in the near future.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization, Data curation, & Visualization: BYL, SBH. Writing-original draft: BYL. Writing-review & editing: SBH.