Abstract
- Since the first successful lung transplantation in 1983, there have been many advances in the field. Nevertheless, the latest data from the International Society for Heart and Lung Transplantation revealed that the risk of death from transplantation is 9%. Various aspects of postoperative management, including mechanical ventilation, could affect intensive care unit stay, hospital stay, and immediate postoperative morbidity and mortality. Complications such as reperfusion injury, graft rejection, infection, and dehiscence of anastomosis increase fatal adverse side effects immediately after surgery. In this article, we review the possible immediate complications after lung transplantation and summarize current knowledge on prevention and treatment.
-
Keywords: critical care; immunosuppression; lung transplantation; postoperative care; postoperative
complications; preventive care; transplant rejection; transplantation immunology
INTRODUCTION
Immediate postoperative management of lung transplant recipients consists of ventilator support and weaning, sedation and pain control, meticulous fluid management, close hemodynamic monitoring, primary graft dysfunction (PGD) or other organ dysfunction, such as acute kidney injury or heart failure. Lung transplantation is a major operation, and various technical surgical complications may occur in the early postoperative period. In order to ease a patient’s return to normal daily life after lung transplantation, rehabilitation and education should be added to the postoperative care plan. However, here we will only cover the immediate postoperative complications and the strategies for the prevention of various complications.
IMMEDIATE POSTOPERATIVE MANAGEMENT OF LUNG TRANSPLANTATION
Medical Complications
Primary graft dysfunction
PGD after lung transplantation represents the multifactorial injury of the transplanted lung that occurs within first 72 hours after transplantation. PGD is the preferred term and is sometimes referred to as “ischemia-reperfusion injury,” “early graft dysfunction,” and “reimplantation edema.” Hypoxia occurring within the first 72 hours after lung transplantation and diffuse opaque radiographic appearances of unknown cause are called PGD. A typical histologic finding is diffuse alveolar damage. The definition proposed by the International Society for Heart and Lung Transplantation (ISHLT) in 2016 is shown in Table 1 [1].
The severity of PGD is evaluated based on the oxygenated artery fraction (PaO2/FiO2 ratio, PF ratio), and if there is no diffuse pulmonary edema on chest X-ray, all PF ratios are considered to be grade 0. The PF ratio is ideally measured at a positive end-expiratory pressure (PEEP) of 5 cm H2O at FiO2 of 1.0 and may need correction at elevated altitudes. All measurements of PGD are performed four times, and reperfusion time of the second lung (T0), 24 hours, 48 hours, and 72 hours (T24, T48, T72). The PGD score at 72 hours may be the most important time-point for predicting survival [2]. In terms of treatment and prognosis, most patients who maintain normal pulmonary artery pressure, usually of PGD grade 2 or lower, could improve with conventional therapy, such as a protective lung ventilation strategy and conservative fluid management. Inhaled nitric oxide (iNO) may be used in patients with severe PGD (grade 3) whose oxygenation is not improving, despite conservative treatment and the maintenance of pulmonary arterial hypertension, although the effect of iNO has not yet been proven [3,4]. However, there is no evidence that the use of iNO prevents PGD [5]. For severe PGD patients not responding to iNO and general treatment, it is advisable to initiate extracorporeal membrane oxygenation (ECMO) within 24 hours of severe PGD, and most patients will improve with venovenous ECMO [6-8]. Prognosis is poor when retransplantation for PGD, and most transplantation centers try to avoid retransplantation [9,10]. PGD is considered a risk factor for chronic lung allograft dysfunction (CLAD), reoperation rate, longer ventilator application, longer intensive care unit (ICU) stay, and higher rate of renal replacement therapy; in principle, it is best to prevent PGD rather than to treat it [11-15]. The selection of adequate donor lungs and optimization of preservation, storage, and intraoperative efforts to reduce ischemia-reperfusion injury is the best strategy to prevent PGD.
Acute rejection
Acute rejection in lung transplantation is still a problem despite aggressive immunosuppressive regimens. Acute rejection is relatively common, especially in the first post-transplant year. International registry data have reported the percentage of acute rejection as approximately 28% [16] and Korean registry data have reported approximately 21% [17]. The problems of acute rejection include not only early graft loss and mortality but also initiation of CLAD.
Clinical manifestations tend to be nonspecific, including cough with or without sputum, dyspnea, mild fever, malaise, or pleural effusion without definite evidence of infection or other causes [18]. Suspicion of acute rejection is important because there are no specific diagnostic tools. Chest X-ray or high-resolution computed tomography (CT) findings are also nonspecific, consisting of bilateral ground glass opacities of lower lobe predominance, interlobular septal thickening without consolidation, atelectasis, or cardiomegaly [19,20].
Acute rejection can be categorized in acute cellular rejection (ACR) and acute antibody-mediated rejection (AMR). The findings of perivascular and peribronchial lymphocytic infiltration in transbronchial biopsy confirm ACR. Prompt management of ACR is important, and treatment generally consists of a 3-day, high-dose, intravenous administration of methylprednisolone with optimizing immunosuppression [18]. AMR is related to antibodies in lung allografts, and this concept evolved as concept of CLAD. The lack of standardized data available for the establishment of diagnostic criteria and proper treatments for AMR have been the greatest difficulties. Therefore, ISHLT published a consensus document on pulmonary AMR which showed that measurable allograft dysfunction and the presence of circulating donor-specific antibodies and positive C4d peritubular capillary staining should be considered diagnostic of AMR [21,22]. Treatment options for AMR include intravenous immunoglobulin, plasmapheresis, or anti-CD 20 monoclonal antibodies; however, the treatment of AMR is still challenging [18]. The response to treatment of AMR can be complete, partial, stabilized or there may be no response to treatment [22].
Postoperative infection
Infectious complications represent one of the most important causes of adverse outcomes in lung transplantation. Compared with other solid organ transplants, reasons for the high susceptibility to infection unique to lung transplantation are the continuous and direct exposure of the lung to environmental pathogens, denervation of the allograft—which destroys host defense mechanisms, such as the cough reflex—and the transmission of infection from the donor lung [23].
Surgical Complications
Postoperative hemorrhage
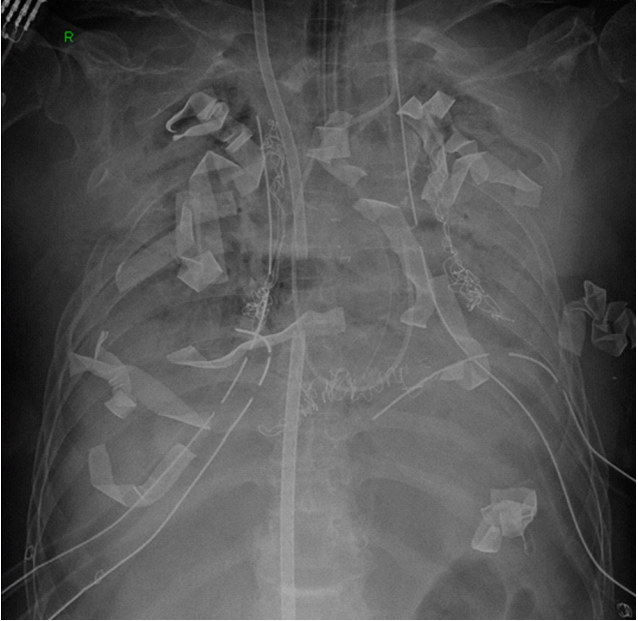
Postoperative hemorrhage was once one of the most common complications (Figure 1). In the early lung transplantation program, hemorrhage was a common complication, requiring about 25% of reoperation patients after cardiopulmonary bypass, en bloc bilateral lung transplantation. However, posterolateral thoracotomy for single lung transplantation and clamshell incision for bilateral lung transplantation have been effective in securing vision and reducing bleeding. In addition, ECMO for intraoperative hemodynamic support, instead of the conventional cardiopulmonary bypass, reduced the amount of intraoperative and postoperative bleeding by decreasing the dose of heparin [24,25].
Large airway complications
Airway complications are major causes that deteriorate the clinical outcomes of recipients. There are several factors involved in large airway complications that occur after lung transplantation, such as interruption of bronchial circulation, airway colonization and infection, and immune responses to airways. Bronchial stenosis is the most frequent airway complication and is usually seen within 2 to 9 months after transplant, with an incidence of between 1.6% and 32% [26]. It is mostly observed at the surgical anastomosis and, on occasion, can occur distally. The right side is more frequently affected. Bronchial stenosis may be asymptomatic or show symptoms of declining expiratory flows, dyspnea, cough, or pneumonia. Stenosis is diagnosed by visual inspection with serial flexible bronchoscopy. Chest CT is useful for characterizing stenosis. Several interventions, including ballooning, inserting stents, and surgical brachioplasty have been used to improve symptoms [27,28]. Bronchial necrosis and dehiscence is a rare complication with an incidence of between 1% and 10% and is associated with high morbidity and mortality, often secondary to sepsis. Necrosis starts at the mucosa and is visible by flexible bronchoscopy. Necrosis without dehiscence may be managed by conservative care, with antimicrobials and keeping fully expanded lung. Dehiscence starts with signs of pneumomediastinum, subcutaneous emphysema, pneumothorax, and new onset of air leakage (Figure 2). A self-expanding metal stent can be applied to stimulate granulation tissue and serve as a scaffold for healing [29]. However, in case of failure, surgical repair or pneumonectomy should be considered. Exophytic granulation tissue narrowing the airway lumen by 25% or more may cause respiratory symptoms. Concurrent infection with aspergillus may increase granulation [30]. Although recurrence is common, debridement or brachytherapy have been effective in relieving symptoms.
Vascular anastomotic complications
Vascular anastomotic complications occur in 1.75% of patients after lung transplantation [31]. Pulmonary artery and atrium anastomosis both can be involved. Donor-recipient size mismatch, the anastomotic technique, twisting or thrombosis may cause these problems. Complications involving the pulmonary artery are more common (Figure 3). Poor orientation of the pulmonary artery and narrowing or kinking due to excessive length may cause these complications. Vascular complications should be suspected when the patient shows unexplained hypoxemia or pulmonary edema. Chest CT or perfusion scan should be considered. If diagnosed, surgical revision or intervention, including stenting or ballooning, can be applied. For the early detection of vascular complications in the operating room, transesophageal echocardiography is useful.
Postoperative Management
Monitoring
Postoperative monitoring in ICUs is on a continuum with intraoperative monitoring. Monitoring in the ICU is essential for the diagnosis of the clinical condition of a patient that can change rapidly and for the planning of future treatment. Like most other centers, our center continues to use pulmonary arterial catheter, electrocardiogram, pulse oximetry, manometer, and transthoracic echocardiography immediately after surgery. Unlike the operating room, postoperative patients in the ICU undergo application of an invasive monitoring device for a few days after surgery. As such, medical staff should be very cautious about the chance of infection associated with invasive monitoring devices. Furthermore, intensivists should always be careful that side effects, such as catheter removal, do not occur, because the patients move, unlike patients during surgery.
Mechanical ventilation care and ventilator weaning
Major respiratory complications (e.g., atelectasis, pneumonia, or respiratory failure) were consistently reported in 15% to 40% of patients within the first 3 days after thoracic surgery [32,33]. The onset of these complications may be associated with a unique pattern of recovery of pulmonary function after open thoracotomy, which presents with an initial 72-hour postoperative delay not seen in other major surgery. Previous studies have shown that active pain control, such as thoracic epidural, may improve postoperative respiratory function [32]. The goal of immediate ventilatory care is to reduce respiratory acidosis and maintain adequate oxygenation. We do not routinely monitor end-tidal CO2 but apply it, if necessary. Patients are usually kept on mechanical ventilation on the day of surgery for monitoring because of the risk of reperfusion injury. Chest X-ray and arterial blood gas analysis are performed at least twice a day to observe the initial changes closely. There is no evidence for which mode is the best for postoperative ventilator setting, but controlled ventilation is preferred to prevent barotrauma on the bronchial anastomosis site and to limit the plateau pressure below 35 mmHg [34]. All recipients are ventilated using a lung-protective ventilation strategy, regardless of the nature of the primary disease (emphysema, pulmonary hypertension or interstitial lung disease) or the type of transplantation (bilateral versus single). Protective mechanical ventilation includes PEEP and low tidal volume (6 to 8 mL/ideal body weight) with permissive hypercapnia. There are no large-scale randomized trials of lung-protective ventilation strategies for patients undergoing lung transplantation. PGD within 72 hours after lung transplantation is similar to acute respiratory distress syndrome (ARDS) clinically and histologically, and lung-protective ventilation strategies are known to be beneficial to ARDS patients. Therefore, most physicians apply a lung-protective ventilation strategy to lung recipients. In a recent multinational survey-based study, most respondents in 18 countries reported using lung-protective ventilation strategies in lung recipients. Low tidal volumes were frequently chosen based on recipient characteristics. On the other hand, the characteristics of donors are not often considered [35].
The PEEP level is selected according to the presence or absence of bronchial fistula [36]. In the presence of bronchial fistula, ZEEP (zero end expiratory pressure) is used, and in recipients without bronchial fistula, different levels of PEEP are applied. PEEP should be set to maximize alveolar recruitment while avoiding over-distention. If the patient is hemodynamically stable and oxygenation is improved adequately (SpO2 >90%, while receiving a FiO2 <0.5 and PEEP <5 cmH2O), mechanical ventilator weaning should be attempted as soon as possible. A 1-hour t-tube challenge test is a convenient and economical way to assess the possibility of weaning success and to predict potential complications associated with spontaneous breathing trials.
Sedation and pain control
In the latest guidelines for sedation in the ICU, benzodiazepine is no longer recommended as a sole sedative, but it is not known which sedative other than benzodiazepine is the best [37]. Similarly, there is no evidence on which combination of sedatives and analgesics has the best prognosis in patients undergoing lung transplantation. According to a recent multicenter survey, more than half of respondents answered that propofol in combination with opiates were preferred over other combinations, such as opiates with intermittent benzodiazepine. However, approximately 40% reported that there is no official drug policy on sedation and analgesia for the transplanted patient [38]. Recently, the use of dexmedetomidine, a selective alpha agonist, has been increasing in ICUs. In many previous studies, it was found that dexmedetomidine can reduce the incidence of postoperative delirium for adult cardiac and non-cardiac surgical patients [39]. In our center, the use of dexmedetomidine during spontaneous breathing trials is increasing in lung-transplanted patients. Dexmedetomidine produces less respiratory depression than other sedatives and has antianxiety effects and small analgesic effects in addition to its sedative properties, so it can be used safely even after extubation.
Postoperative dysrhythmia
Cardiovascular adverse events, including myocardial infarction and dysrhythmia, are frequent in the immediate postoperative period. Atrial fibrillation has been found in about 40% of recipients and generally responds well to conventional treatments, such as calcium channel blockers, beta blockers and amiodarone. The elderly patients, and the presence of idiopathic pulmonary fibrosis, known coronary disease, enlarged left atrium, and the use of postoperative vasopressors increase the risk of postoperative atrial fibrillation [40]. In our institution, transesophageal echocardiography is performed in patients with postoperative cardiovascular adverse events or preoperative cardiac comorbidities before ventilator weaning. Antithrombotic agents are not routinely used because of the risk of bleeding immediately after surgery, but they may be used with caution when dysregulated arrhythmia persists.
Fluid management
Fluid management may influence the degree of lung dysfunction. After lung transplantation, ischemia-reperfusion injury increases pulmonary vascular permeability and decreases lymphatic drainage, resulting in some degree of pulmonary edema [41]. To decrease the postoperative pulmonary vascular burden, we perform intravenous fluid therapy very restrictively and cautiously, to maintain adequate urine output (0.5 to 1 ml/kg/hr or more), oxygen saturation level (SpO2 >95% or more) and mean blood pressure (65 mmHg or more) [42]. Dynamic parameters, such as pulmonary capillary wedge or central venous pressure, have traditionally been used to assess adequate fluid balance in the immediate postoperative patients. Recently, various minimally invasive hemodynamic monitoring devices, including pulse pressure/stroke volume variation [43], esophageal doppler [44], and extravascular lung water measurement [45], were evaluated for optimizing perioperative fluid therapy for major surgery. One thing that is clear is that excessive fluid resuscitation or inotropic support resulting in a hyperdynamic right ventricle and the exacerbation of lung reperfusion injury should be avoided [46].
Immunosuppressant use and monitoring
Immunosuppressants are important for the prevention of graft rejection. Immunosuppressants can be classified as “induction” and “maintenance.” The purpose of induction therapy is to reinforce early immunosuppression and to allow for a less toxic maintenance immunosuppressant going forward [47]. However, induction therapy is still controversial in patients undergoing lung transplantation, and registry data shows that induction therapy is used in approximately 60% of transplantation centers [16]. Polyclonal antithymocyte globulin, interleukin-2R antagonists or alemtuzumab can be used for induction therapy [16].
The use of maintenance immunosuppressants in lung transplantation generally involves a three-drug regimen and the exact composition can vary by transplantation center. The three-drug regimen includes a calcineurin inhibitor (CNI; tacrolimus or cyclosporine), a corticosteroid and a nucleotide blocking agent (mycophenolate mofetil or azathioprine). Recently, the use of a combination of tacrolimus, corticosteroid, and mycophenolate has been increasing [16].
In lung transplantation, higher maintenance immunosuppression is needed because lung tissues have an apparently higher immunogenicity. Therefore, therapeutic drug monitoring (TDM) is important for treatment individualization and TDM for CNI is always needed in lung transplantation. The target blood concentration for the CNI can be different depending on the center. Moreover, minimizing the variability of blood levels is also needed adding to the maintaining the high mean CNI level for reducing the risk of allograft rejection [48]. Variability in blood concentration can be related to non-adherence with drug guideline, interacting medications (such as some types of anti-fungal agents or rifampin), differences in the cytochrome P450 enzyme system, changes in the fat content of meals and changes in gastrointestinal motility [48,49].
Infection control: immunization
Candidates for lung transplantation should have their vaccines updated as early as possible before transplantation, because antibody responses to vaccines are decreased during organ failure, and are significantly reduced after transplantation by mycophenolate mofetil treatment and advanced age. The optimal timing to begin posttransplant vaccination is 6 months after transplantation but should be individualized for each patient. Lung transplantation candidates without hepatitis A or B antibodies are vaccinated prior to transplantation. Before lung transplantation, a 13-valent protein-conjugated pneumococcal vaccine is used for priming, followed 8 weeks later by a 23-valent polysaccharide vaccine. Candidates that have not received the tetanus, diphtheria, pertussis (Tdap) vaccine or are unaware of their Tdap vaccine status are administered the Tdap vaccine and a tetanus-diphtheria booster every 10 years. All lung transplantation candidates and recipients are advised to receive annual influenza vaccinations.
Infection control: prophylactic strategies for postoperative infections
For the prevention of postoperative infections, antibacterial, antifungal, and antiviral prophylaxis is administered. Intraoperative antibiotic prophylaxis includes cefepime for Gram-negative coverage, and vancomycin or teicoplanin for Gram-positive coverage. The cefepime and vancomycin or teicoplanin are typically discontinued or de-escalated after 5 days, once all intraoperative cultures are finalized as negative.
Regardless of the cytomegalovirus (CMV) serostatus of recipients and donors, intravenous ganciclovir is given for antiviral prophylaxis at a dosage of 5 mg/kg every 24 hours after lung transplantation. If the recipient can tolerate oral intake, he or she is transitioned to valganciclovir at a dose of 900 mg orally daily. The total duration of antiviral prophylaxis is 6 months, but in patients who are CMV IgG negative with a CMV immunoglobulin G-positive donor, CMV prophylaxis is considered for lifetime as tolerated. Ganciclovir and valganciclovir dosing is adjusted based on renal function.
Fungal infections, especially by Aspergillus species, are very common. One frequent site of infection is the bronchial anastomosis. For this reason, most centers recommend some form of fungal prophylaxis at least during the first 3 months after the transplant [50]. Antifungal prophylaxis varies from center to center. Azole antifungals or inhaled formulations of amphotericin B are used, depending on the situation. We use itraconazole for antifungal prophylaxis during the 6 months after transplant.
In solid organ transplant recipients, Pneumocystis jirovecii infection is associated with a high mortality rate, despite an adapted treatment [51,52]. Therefore, P. jirovecii prophylaxis is commonly instituted after all solid organ transplants. There are different guidelines addressing P. jirovecii prophylaxis after solid organ transplants and there is no universal consensus on the optimal duration of prophylaxis. The American Society of Transplantation recommends a general prophylaxis for 6–12 months after solid organ transplants. Lifelong prophylaxis is recommended for patients after lung or small-bowel transplantation, chronic CMV infection, and in all patients with a history of prior P. jirovecii infection [53]. In our center, lifelong trimethoprim/sulfamethoxazole (80/400 mg or 160/800 mg) is administered to prevent P. jirovecii infection after lung transplantation.
Venous thromboembolism prevention
Lung transplant recipients are at increased risk for postoperative venous thromboembolism (VTE: deep vein thrombosis and pulmonary embolism) [54,55]. Most patients are treated with low-dose subcutaneous unfractioned heparin and intermittent pneumatic compression [56], but patients with a history of previous VTE may require more intensive therapy [57]. In general, VTE prevention continues until hospital discharge.
Surveillance bronchoscopy
The role of surveillance bronchoscopy for regular biopsy in asymptomatic patients receiving lung transplants is still controversial and the strategy depends on the center. Schedules of surveillance bronchoscopy are different; however, monthly biopsies are performed in the immediate postoperation period and the interval is increased after 3 months in many centers. There are some reports that the incidence of asymptomatic ACR is up to 25% [58]; therefore, some centers maintain strategies for surveillance bronchoscopy.
CONCLUSIONS
The immediate postoperative period is critical for the management of patients undergoing lung transplantation. PGD, acute rejection, and postoperative infection should be carefully monitored. In addition, it is important to understand that postoperative hemorrhage, large airway complications, and vascular anastomotic complications may occur due to the complexity of the lung transplant procedure itself. Therefore, intraoperative hemodynamic monitoring should be continued in the ICU after surgery. Moreover, based on hemodynamic parameters, meticulous fluid management and mechanical ventilation should be performed. The application and maintenance of immunosuppressants in patients with lung transplantation vary from center to center, but the combination of tacrolimus, corticosteroids, and mycophenolate has increased recently. Controlling the plasma levels of immunosuppressants is very important for reducing allograft rejection. Patients undergoing lung transplantation who are at high risk for infection should be preoperatively vaccinated and given prophylactic antibiotics after surgery. The risk of PTE is higher than in patients after other major surgery, and VTE prevention should be continued during hospitalization. The postoperative critical care management after lung transplantation is very complicated; therefore, a careful approach is important. For these reasons, a multidisciplinary approach is needed, and proper critical care management after lung transplantation is essential to improve outcomes.
KEY MESSAGES
▪ We review the possible immediate complications after lung transplantation.
▪ We summarize current knowledge on prevention and treatment of possible immediate complications after lung transplantation.
NOTES
-
No potential conflict of interest relevant to this article was reported.
Figure 1.Gauzes were packed to control postoperative bleeding.
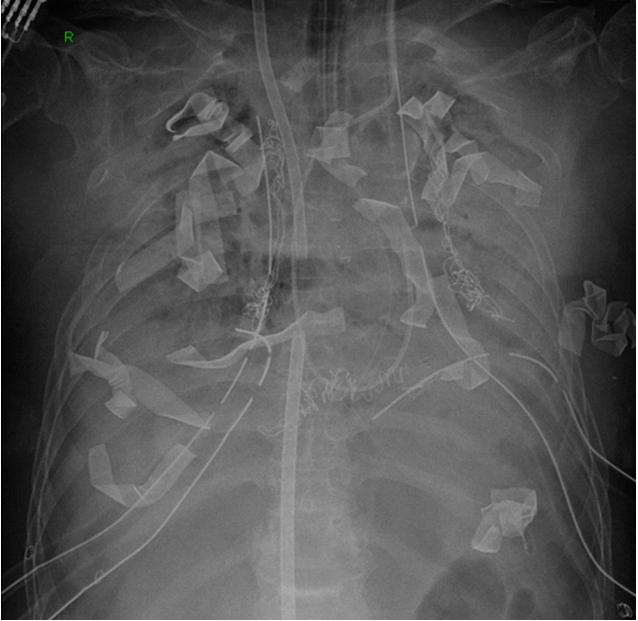
Figure 2.Bronchopleural fistula (BPF). (A) Fiberoptic bronchoscopy (FOB) finding of BPF (arrows). (B) Suddenly developed pneumothorax and subcutaneous emphysema on chest X-ray. (C) FOB finding showing repaired BPF with omental flap. (D) Chest X-ray after BPF repair with omental flap.
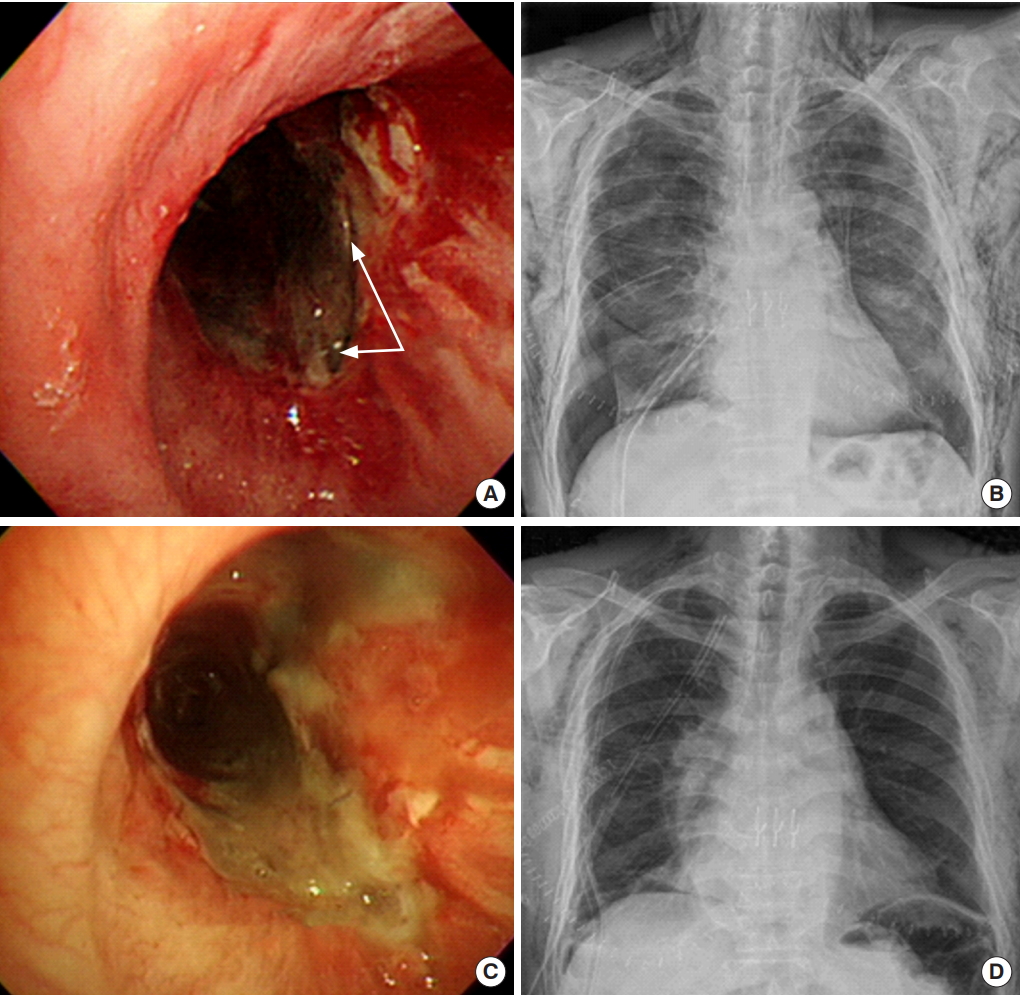
Figure 3.Pulmonary artery stenosis. (A) Pulmonary angiography shows nearly total obstruction (kinking) of left main pulmonary artery. (B) A stent was inserted on left main pulmonary artery and left pulmonary flow was restored.

Table 1.The 2016 International Society for Heart and Lung Transplantation definition of PGD
|
Gradea
|
Pulmonary edema on chest X-ray |
PaO2/FiO2 ratio |
|
PGD grade 0 |
No |
Any |
|
PGD grade 1 |
Yes |
>300 |
|
PGD grade 2 |
Yes |
200–300 |
|
PGD grade 3 |
Yes |
<200 |
References
- 1. Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading-A 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1097-103.ArticlePubMed
- 2. Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34.ArticlePubMedPMC
- 3. Van Raemdonck D, Hartwig MG, Hertz MI, Davis RD, Cypel M, Hayes D Jr, et al. Report of the ISHLT working group on primary lung graft dysfunction part IV: prevention and treatment: a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1121-36.ArticlePubMed
- 4. Pasero D, Martin EL, Davi A, Mascia L, Rinaldi M, Ranieri VM. The effects of inhaled nitric oxide after lung transplantation. Minerva Anestesiol 2010;76:353-61.PubMed
- 5. Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One 2014;9:e92773.ArticlePubMedPMC
- 6. Wigfield CH, Lindsey JD, Steffens TG, Edwards NM, Love RB. Early institution of extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation improves outcome. J Heart Lung Transplant 2007;26:331-8.ArticlePubMed
- 7. Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S, et al. Report of the ISHLT working group on primary lung graft dysfunction part VI: treatment. J Heart Lung Transplant 2005;24:1489-500.ArticlePubMed
- 8. Fiser SM, Kron IL, McLendon Long S, Kaza AK, Kern JA, Tribble CG. Early intervention after severe oxygenation index elevation improves survival following lung transplantation. J Heart Lung Transplant 2001;20:631-6.ArticlePubMed
- 9. Aigner C, Jaksch P, Taghavi S, Lang G, Reza-Hoda MA, Wisser W, et al. Pulmonary retransplantation: is it worth the effort? A long-term analysis of 46 cases. J Heart Lung Transplant 2008;27:60-5.ArticlePubMed
- 10. Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report-2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24.ArticlePubMed
- 11. Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 2002;73:1041-7.ArticlePubMed
- 12. Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and longterm pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11.ArticlePubMed
- 13. Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507-13.ArticlePubMed
- 14. Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg 2008;86:189-95.ArticlePubMedPMC
- 15. Moon S, Park MS, Lee JG, Jung JY, Kang YA, Kim YS, et al. Risk factors and outcome of primary graft dysfunction after lung transplantation in Korea. J Thorac Dis 2016;8:3275-82.ArticlePubMedPMC
- 16. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46.ArticlePubMed
- 17. Korean Organ Transplantation Registry. KOTRY annual data report 2016. Seoul, KOTRY. 2017.
- 18. Fuehner T, Greer M, Welte T, Gottlieb J. The lung transplant patient in the ICU. Curr Opin Crit Care 2012;18:472-8.ArticlePubMed
- 19. Millet B, Higenbottam TW, Flower CD, Stewart S, Wallwork J. The radiographic appearances of infection and acute rejection of the lung after heart-lung transplantation. Am Rev Respir Dis 1989;140:62-7.ArticlePubMed
- 20. Park CH, Paik HC, Haam SJ, Lim BJ, Byun MK, Shin JA, et al. HRCT features of acute rejection in patients with bilateral lung transplantation: the usefulness of lesion distribution. Transplant Proc 2014;46:1511-6.ArticlePubMed
- 21. Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant 2004;4:1562-6.ArticlePubMed
- 22. Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: a consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2016;35:397-406.ArticlePubMed
- 23. Speich R, van der Bij W. Epidemiology and management of infections after lung transplantation. Clin Infect Dis 2001;33 Suppl 1:S58-65.ArticlePubMedPDF
- 24. Machuca TN, Collaud S, Mercier O, Cheung M, Cunningham V, Kim SJ, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7.ArticlePubMed
- 25. Yu WS, Paik HC, Haam SJ, Lee CY, Nam KS, Jung HS, et al. Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes. J Thorac Dis 2016;8:1712-20.ArticlePubMedPMC
- 26. Garfein ES, McGregor CC, Galantowicz ME, Schulman LL. Deleterious effects of telescoped bronchial anastomosis in single and bilateral lung transplantation. Ann Transplant 2000;5:5-11.
- 27. Cho EN, Haam SJ, Kim SY, Chang YS, Paik HC. Anastomotic airway complications after lung transplantation. Yonsei Med J 2015;56:1372-8.ArticlePubMedPMC
- 28. De Gracia J, Culebras M, Alvarez A, Catalán E, De la Rosa D, Maestre J, et al. Bronchoscopic balloon dilatation in the management of bronchial stenosis following lung transplantation. Respir Med 2007;101:27-33.ArticlePubMed
- 29. Mughal MM, Gildea TR, Murthy S, Pettersson G, DeCamp M, Mehta AC. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71.ArticlePubMed
- 30. Mulligan MS. Endoscopic management of airway complications after lung transplantation. Chest Surg Clin N Am 2001;11:907-15.PubMed
- 31. Clark SC, Levine AJ, Hasan A, Hilton CJ, Forty J, Dark JH. Vascular complications of lung transplantation. Ann Thorac Surg 1996;61:1079-82.ArticlePubMed
- 32. Licker MJ, Widikker I, Robert J, Frey JG, Spiliopoulos A, Ellenberger C, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7.ArticlePubMed
- 33. Busch E, Verazin G, Antkowiak JG, Driscoll D, Takita H. Pulmonary complications in patients undergoing thoracotomy for lung carcinoma. Chest 1994;105:760-6.ArticlePubMed
- 34. Lau CL, Patterson GA, Palmer SM. Critical care aspects of lung transplantation. J Intensive Care Med 2004;19:83-104.ArticlePubMed
- 35. Beer A, Reed RM, Bölükbas S, Budev M, Chaux G, Zamora MR, et al. Mechanical ventilation after lung transplantation. An international survey of practices and preferences. Ann Am Thorac Soc 2014;11:546-53.ArticlePubMed
- 36. Shekar K, Foot C, Fraser J, Ziegenfuss M, Hopkins P, Windsor M. Bronchopleural fistula: an update for intensivists. J Crit Care 2010;25:47-55.ArticlePubMed
- 37. Barr J, Pandharipande PP. The pain, agitation, and delirium care bundle: synergistic benefits of implementing the 2013 Pain, Agitation, and Delirium Guidelines in an integrated and interdisciplinary fashion. Crit Care Med 2013;41(9 Suppl 1):S99-115.ArticlePubMed
- 38. King CS, Valentine V, Cattamanchi A, Franco-Palacios D, Shlobin OA, Brown AW, et al. Early postoperative management after lung transplantation: results of an international survey. Clinical Transplantation 2017;31:e12985. Article
- 39. Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth 2018;121:384-97.ArticlePubMed
- 40. Nielsen TD, Bahnson T, Davis RD, Palmer SM. Atrial fibrillation after pulmonary transplant. Chest 2004;126:496-500.ArticlePubMed
- 41. Kaplan JD, Trulock EP, Cooper JD, Schuster DP. Pulmonary vascular permeability after lung transplantation: a positron emission tomographic study. Am Rev Respir Dis 1992;145(4 Pt 1):954-7.ArticlePubMed
- 42. Evans RG, Naidu B. Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury? Interact Cardiovasc Thorac Surg 2012;15:498-504.ArticlePubMedPMCPDF
- 43. Teboul JL, Monnet X. Pulse pressure variation and ARDS. Minerva Anestesiol 2013;79:398-407.PubMed
- 44. Waldron NH, Miller TE, Thacker JK, Manchester AK, White WD, Nardiello J, et al. A prospective comparison of a noninvasive cardiac output monitor versus esophageal Doppler monitor for goal-directed fluid therapy in colorectal surgery patients. Anesth Analg 2014;118:966-75.ArticlePubMed
- 45. Tran-Dinh A, Augustin P, Dufour G, Lasocki S, Allou N, Thabut G, et al. Evaluation of cardiac index and extravascular lung water after single-lung transplantation using the transpulmonary thermodilution technique by the PiCCO2 device. J Cardiothorac Vasc Anesth 2018;32:1731-5.ArticlePubMed
- 46. Potestio C, Jordan D, Kachulis B. Acute postoperative management after lung transplantation. Best Pract Res Clin Anaesthesiol 2017;31:273-84.ArticlePubMed
- 47. Yeung JC, Keshavjee S. Overview of clinical lung transplantation. Cold Spring Harb Perspect Med 2014;4:a015628. ArticlePubMedPMC
- 48. Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant 2010;14:968-75.ArticlePubMed
- 49. Bucuvalas JC, Ryckman FC, Arya G, Andrew B, Lesko A, Cole CR, et al. A novel approach to managing variation: outpatient therapeutic monitoring of calcineurin inhibitor blood levels in liver transplant recipients. J Pediatr 2005;146:744-50.ArticlePubMed
- 50. Campos S, Caramori M, Teixeira R, Afonso J Jr, Carraro R, Strabelli T, et al. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc 2008;40:822-4.ArticlePubMed
- 51. Gordon SM, LaRosa SP, Kalmadi S, Arroliga AC, Avery RK, Truesdell-LaRosa L, et al. Should prophylaxis for Pneumocystis carinii pneumonia in solid organ transplant recipients ever be discontinued? Clin Infect Dis 1999;28:240-6.ArticlePubMedPDF
- 52. Bourbigot B, Bensoussan T, Garo B, Islam MS, Hardy E, Moal MC, et al. CD4 T-lymphocyte counts as predictors of pneumonia after kidney transplantation. Transplant Proc 1993;25(1 Pt 2):1491-2.PubMed
- 53. Martin SI, Fishman JA, AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:272-9.ArticlePubMed
- 54. Gómez-Hernández MT, Rodríguez-Pérez M, Novoa-Valentín N, Jiménez-López M, Aranda-Alcaide JL, Varela-Simó G. Prevalence of venous thromboembolism in elective thoracic surgery. Arch Bronconeumol 2013;49:297-302.ArticlePubMed
- 55. Kalweit G, Huwer H, Volkmer I, Petzold T, Gams E. Pulmonary embolism: a frequent cause of acute fatality after lung resection. Eur J Cardiothorac Surg 1996;10:242-6.ArticlePubMedPDF
- 56. Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Intermittent pneumatic compression is effective in preventing symptomatic pulmonary embolism after thoracic surgery. Surg Today 2004;34:6-10.ArticlePubMed
- 57. Di Nisio M, Peinemann F, Porreca E, Rutjes AW. Primary prophylaxis for venous thromboembolism in patients undergoing cardiac or thoracic surgery. Cochrane Database Syst Rev 2015;19:CD009658. Article
- 58. McWilliams TJ, Williams TJ, Whitford HM, Snell GI. Surveillance bronchoscopy in lung transplant recipients: risk versus benefit. J Heart Lung Transplant 2008;27:1203-9.ArticlePubMed
Citations
Citations to this article as recorded by

- Aspergillus Galactomannan Titer as a Diagnostic Marker of Invasive Pulmonary Aspergillosis in Lung Transplant Recipients: A Single-Center Retrospective Cohort Study
Eun-Young Kim, Seung-Hyun Yong, Min-Dong Sung, A-La Woo, Young-Mok Park, Ha-Eun Kim, Su-Jin Jung, Song-Yee Kim, Jin-Gu Lee, Young-Sam Kim, Hyo-Chae Paik, Moo-Suk Park
Journal of Fungi.2023; 9(5): 527. CrossRef - Nontuberculous mycobacterial infection after lung transplantation: A single-center experience in South Korea
Youngmok Park, Nam Eun Kim, Se Hyun Kwak, Moo Suk Park, Su Jin Jeong, Jin Gu Lee, Hyo Chae Paik, Song Yee Kim, Young Ae Kang
Journal of Microbiology, Immunology and Infection.2022; 55(1): 123. CrossRef - Medical Complications of Lung Transplantation
Moo Suk Park
Journal of Chest Surgery.2022; 55(4): 338. CrossRef - Roles of electrical impedance tomography in lung transplantation
Hui Jiang, Yijiao Han, Xia Zheng, Qiang Fang
Frontiers in Physiology.2022;[Epub] CrossRef - Perioperative anidulafungin combined with triazole prophylaxis for the prevention of early invasive candidiasis in lung transplant recipients
Emily Sartain, Kelly Schoeppler, Barrett Crowther, Joshua B. Smith, Maheen Z. Abidi, Todd J. Grazia, Mark Steele, Terri Gleason, Krista Porter, Alice Gray
Transplant Infectious Disease.2021;[Epub] CrossRef - The Prediction and Prognosis of Fungal Infection in Lung Transplant Recipients—A Retrospective Cohort Study in South Korea
Yae-Jee Baek, Yun-Suk Cho, Moo-Hyun Kim, Jong-Hoon Hyun, Yu-Jin Sohn, Song-Yee Kim, Su-Jin Jeong, Moo-Suk Park, Jin-Gu Lee, Hyo-Chae Paik
Journal of Fungi.2021; 7(8): 639. CrossRef - Panel-Reactive and Donor-Specific Antibodies before Lung Transplantation can Affect Outcomes in Korean Patients Receiving Lung Transplantation
Sung Woo Moon, Moo Suk Park, Jin Gu Lee, Hyo Chae Paik, Young Tae Kim, Hyun Joo Lee, Samina Park, Sun Mi Choi, Do Hyung Kim, Woo Hyun Cho, Hye Ju Yeo, Seung-il Park, Se Hoon Choi, Sang-Bum Hong, Tae Sun Shim, Kyung-Wook Jo, Kyeongman Jeon, Byeong-Ho Jeong
Yonsei Medical Journal.2020; 61(7): 606. CrossRef - A proof-of principal study using phase-contrast imaging for the detection of large airway pathologies after lung transplantation
Stephan Umkehrer, Carmela Morrone, Julien Dinkel, Laura Aigner, Maximilian F. Reiser, Julia Herzen, Ali Ö. Yildirim, Franz Pfeiffer, Katharina Hellbach
Scientific Reports.2020;[Epub] CrossRef
 , Su Jin Jeong2
, Su Jin Jeong2 , Jin Gu Lee3
, Jin Gu Lee3 , Moo Suk Park1
, Moo Suk Park1 , Hyo Chae Paik3
, Hyo Chae Paik3 , Sungwon Na4
, Sungwon Na4 , Jeongmin Kim4
, Jeongmin Kim4








 KSCCM
KSCCM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite