Articles
- Page Path
- HOME > Acute Crit Care > Volume 36(1); 2021 > Article
-
Image in Critical Care
Pulmonary Pneumothorax and pulmonary air leaks as ventilator-induced injuries in COVID-19 -
Gabriele Martelli
 , Ivo Tiberio
, Ivo Tiberio -
Acute and Critical Care 2021;36(1):75-77.
DOI: https://doi.org/10.4266/acc.2020.00955
Published online: January 13, 2021
Intensive Care Unit U.O.C. Anestesia e Rianimazione, Department of Surgery, University Hospital of Padua, Padua, Italy
- Corresponding author Gabriele Martelli Intensive Care Unit U.O.C. Anestesia e Rianimazione, Department of Surgery, University Hospital of Padua, Via Giustiniani 2 35128 Padua, Italy Tel: +39-0498212745 Fax: +39-0498218269 E-mail: gabriele.martelli@aopd.veneto.it
• Received: November 17, 2020 • Revised: December 10, 2020 • Accepted: December 21, 2020
Copyright © 2021 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 7,195 Views
- 208 Download
- 1 Web of Science
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conceptualization, Visualization, Writing–original draft: GM. Writing–review & editing: all author.
NOTES
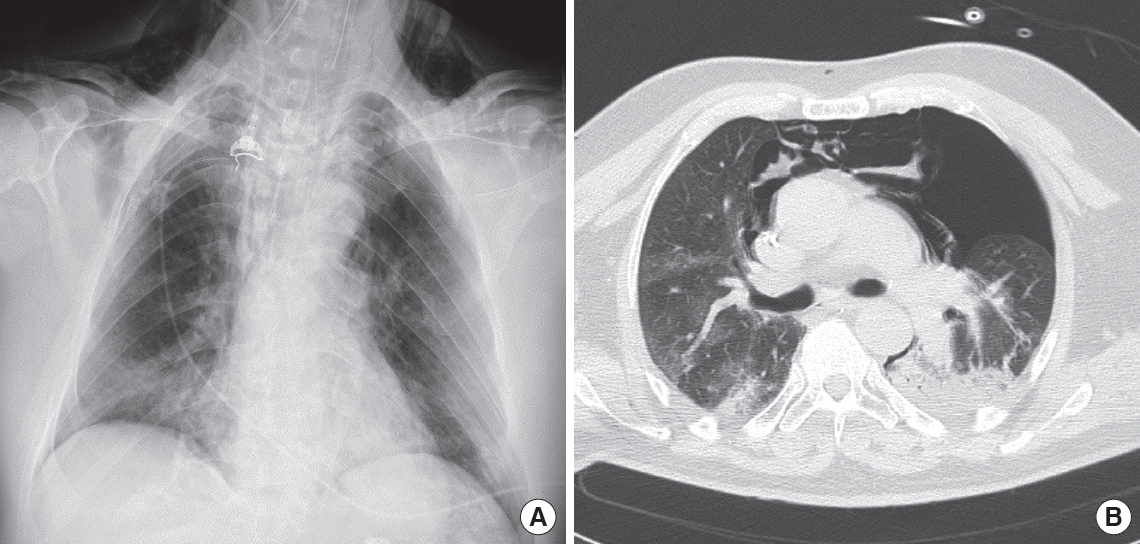
Figure 1.Chest X-ray (A) and computed tomography thoracic scan (B) of a 59-year-old male coronavirus disease 2019 (COVID-19) patient after 3 days of invasive ventilation. Ventilation occurred in pressure-control mode with the following parameters: peak inspiratory pressure, 27 cm H2O; positive end-expiratory pressure, 12 cm H2O; fraction of inspired oxygen, 0.6; inspiratory to expiratory ratio, 1:2; and respiratory rate, 16. The last measurement prior to the occurrence of pneumothorax was a plateau pressure of 25 cm H2O and static compliance of 43 L/cm H2O. Bilateral inhomogeneous parenchyma and consolidative aspects of the left lung were noted. The patient developed left pneumothorax and pneumomediastinum. On chest X-ray, subcutaneous emphysema is evident.
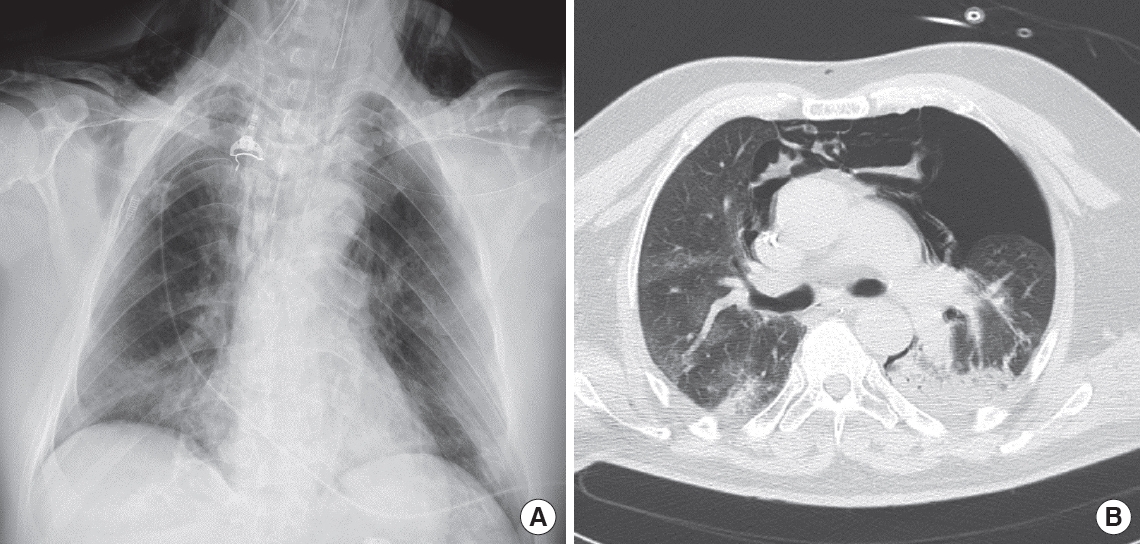

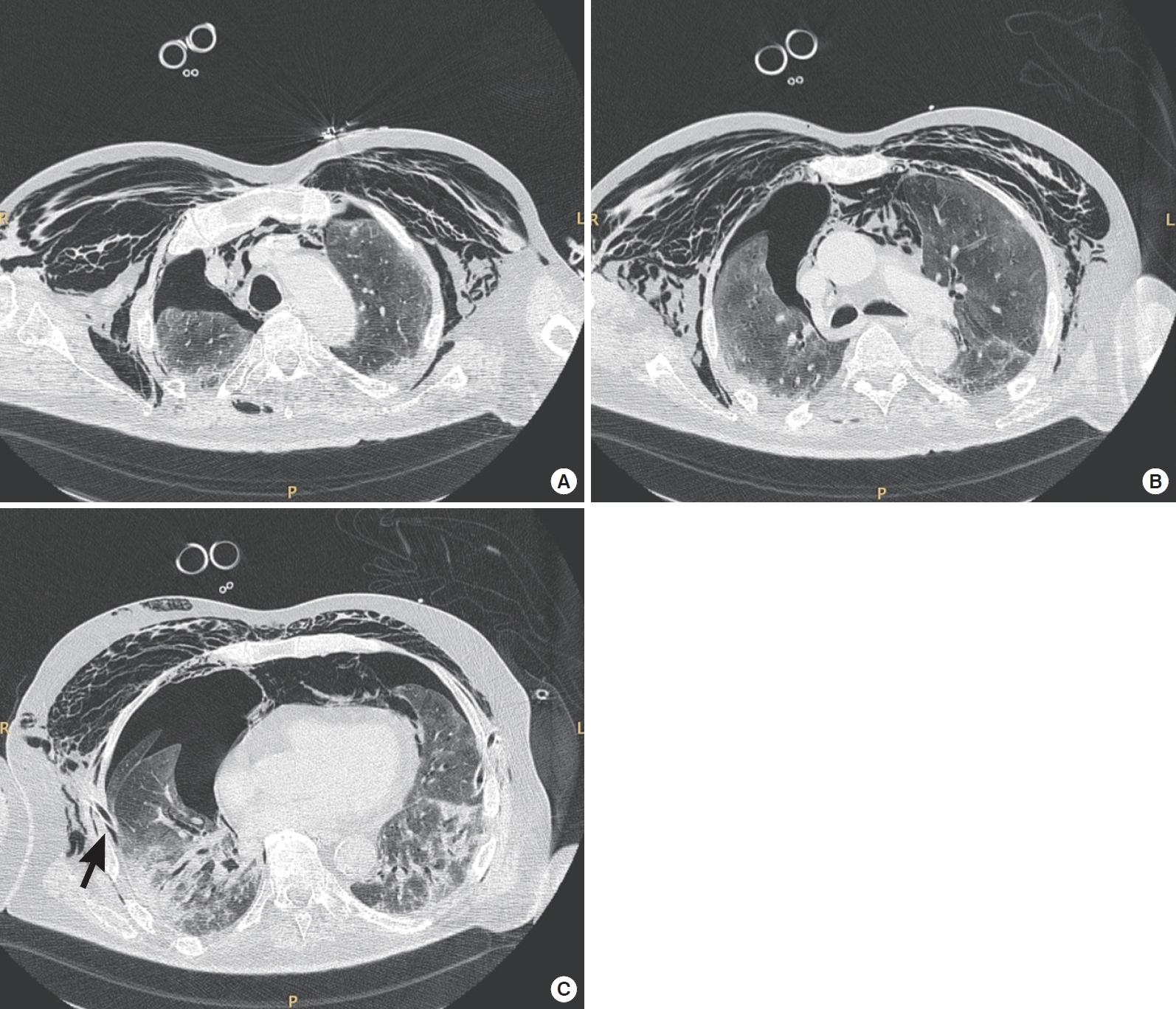
Figure 2.Thoracic computed tomography axial scans of a 75-year-old coronavirus disease 2019 (COVID-19) patient with moderate acute respiratory distress syndrome. The scans were obtained at (A) upper, (B) middle, and (C) lower thoracic level. The patient was receiving pressure support ventilation (pressure support, 14 cm H2O; positive end-expiratory pressure, 10 cm H2O; fraction of inspired oxygen, 0.75; and mean respiratory rate, 18). These scans revealed a failure of lung re-expansion after right thoracic drainage (black arrow) and persistence of pneumothorax, pneumomediastinum, and subcutaneous emphysema.
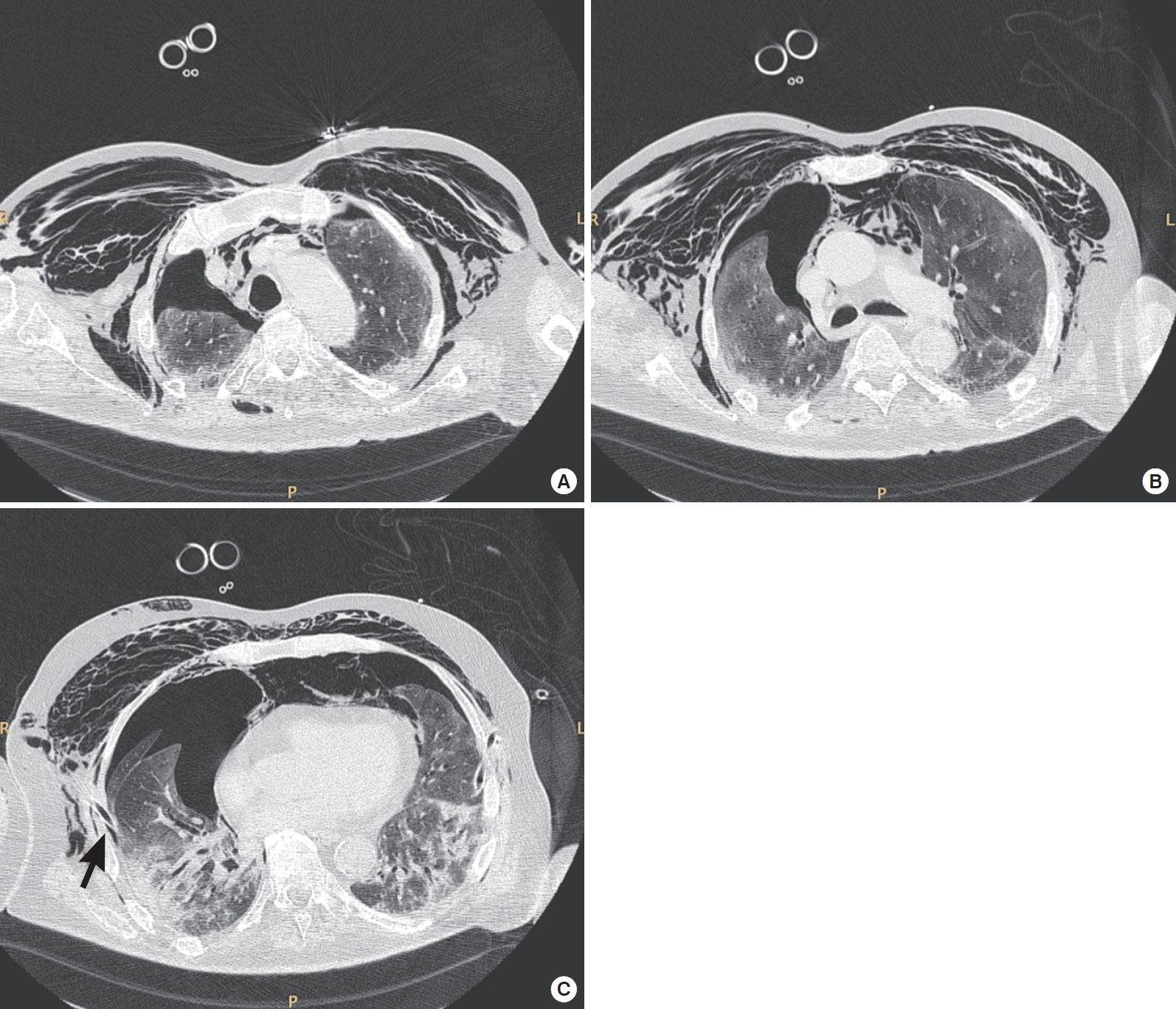

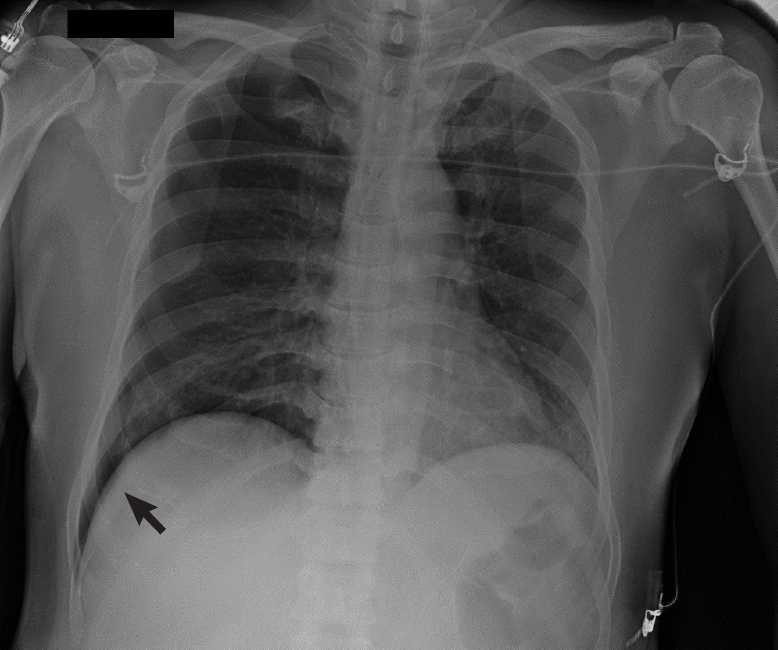
Figure 3.Chest X-ray of a 40-year-old male coronavirus disease 2019 (COVID-19) patient. Right pneumothorax of 30 mm. “Deep sulcus sign” was noted (black arrow). This patient developed pneumothorax after a cycle of non-invasive ventilation with a helmet interface. Ventilation was set at pressure support, 8 cm H2O; positive end-expiratory pressure, 10 cm H2O; and fraction of inspired oxygen, 0.55.


- 1. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13.ArticlePubMedPMC
- 2. Benson JC, Carlson ML, Lane JI. MRI of the internal auditory canal, labyrinth, and middle ear: how we do it. Radiology 2020;297:252-65.ArticlePubMed
- 3. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020;396:320-32.ArticlePubMedPMC
- 4. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608.ArticlePubMed
- 5. Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, González C, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care 2020;24:494. ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite