Articles
- Page Path
- HOME > Acute Crit Care > Volume 37(2); 2022 > Article
- Original Article The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation
-
Jae Ha Lee1
 , Jin Han Park1
, Jin Han Park1 , Ji Hoon Jang2
, Ji Hoon Jang2 , Se Hun Kim3
, Se Hun Kim3 , Sung Yong Hong4
, Sung Yong Hong4 , Woon Heo4
, Woon Heo4 , Dong-Hwan Lee5
, Dong-Hwan Lee5 , Hye Sook Choi6
, Hye Sook Choi6 , Ki Hoon Kim7
, Ki Hoon Kim7 , Hang-Jea Jang1
, Hang-Jea Jang1
-
Acute and Critical Care 2022;37(2):177-184.
DOI: https://doi.org/10.4266/acc.2021.01312
Published online: February 17, 2022
1Division of Pulmonology, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
2Division of Pulmonology and Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
3Department of Anesthesiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
4Department of Thoracic and Cardiovascular Surgery, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
5Department of Clinical Pharmacology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
6Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University College of Medicine, Seoul, Korea
7Department of General Surgery, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- Corresponding author: Hang-Jea Jang Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 48108, Korea Tel: +82-51-797-0100, Fax: +82-51-797-3009, E-mail: okabango21@gmail.com
Copyright © 2022 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Anticoagulation during extracorporeal membrane oxygenation (ECMO) usually is required to prevent thrombosis. The aim of this study was to investigate the usefulness of nafamostat mesilate (NM) as a regional anticoagulant during veno-arterial ECMO (VA-ECMO) treatment.
-
Methods
- We retrospectively reviewed the medical records of 16 patients receiving VA-ECMO and NM from January 2017 to June 2020 at Haeundae Paik Hospital. We compared clinical and laboratory data, including activated partial thromboplastin time (aPTT), which was measured simultaneously in patients and the ECMO site, to estimate the efficacy of regional anticoagulation.
-
Results
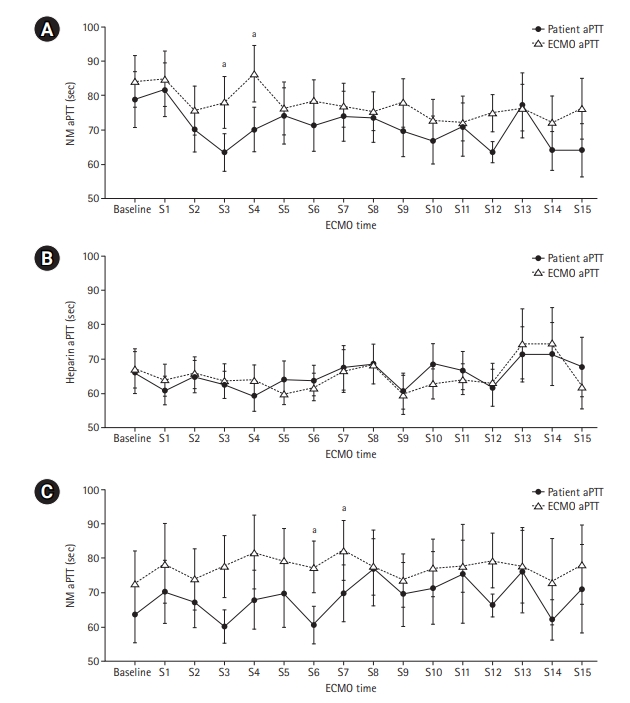
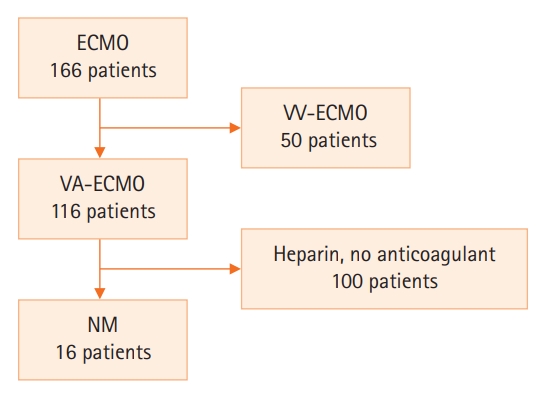
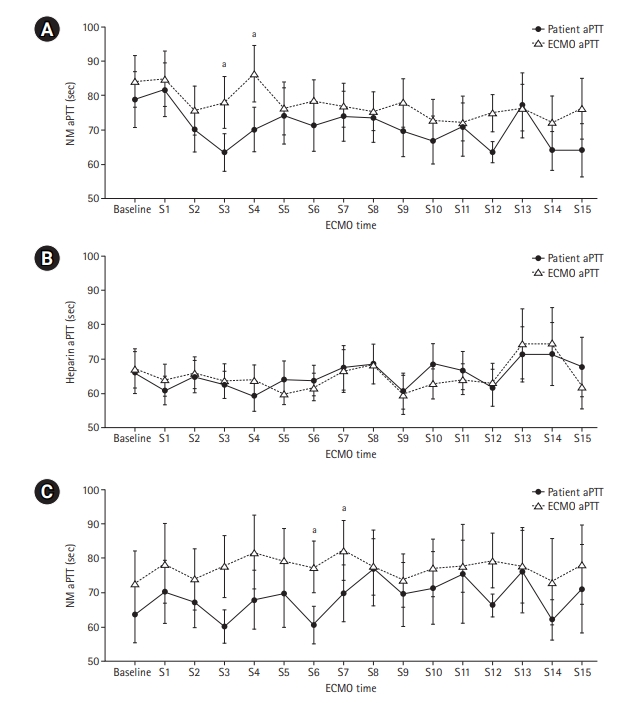
- The median patient age was 68.5 years, and 56.3% of patients were men. Cardiovascular disease was the most common primary disease (75.0%) requiring ECMO treatment, followed by respiratory disease (12.5%). The median duration of ECMO treatment was 7.5 days. Among 16 patients, seven were switched to NM after first using heparin as an anticoagulation agent, and nine received only NM. When comparing aPTT values in the NM group between patients and the ECMO site, that in patients was significantly lower than that at the ECMO site (73.57 vs. 79.25 seconds; P=0.010); in contrast, no difference was observed in the heparin group.
-
Conclusions
- NM showed efficacy as a regional anticoagulation method by sustaining a lower aPTT value compared to that measured at the ECMO site. NM should be considered as a safer regional anticoagulation method in VA-ECMO for patients at high risk of bleeding.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
KEY MESSAGES
-
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conceptualization: JHL, HJJ. Data curation: JHP, JHJ, SHK, WH, KHK. Formal analysis: SYH, SHK, DHL, KHK. Methodology: SYH, SHK, DHL, KHK. Visualization: JHL, SYH. Writing–original draft: JHL, HSC, JHP. Writing–review & editing: all authors.
NOTES
Acknowledgments
| Characteristics | Value (n=16) |
|---|---|
| Age (yr) | 68.5 (53.5–73.0) |
| Male | 9 (56.3) |
| Height (m) | 1.65 (1.61–1.70) |
| Body weight (kg) | 58.75 (54.13–75.83) |
| Predicted body weight (kg) | 58.00 (52.75–61.75) |
| Primary disease | |
| Respiratory disease | 2 (12.5) |
| Cardiovascular disease | 12 (75.0) |
| Gastrointestinal disease | 1 (6.3) |
| Renal disease | 1 (6.3) |
| APACHE II score | 24.50 (18.50–27.75) |
| SAPS III score | 60.00 (48.25–73.00) |
| Shocka | 16 (100.0) |
| CRRT | 9 (56.3) |
| Serum CRP (mg/dl) | 4.27 (0.87–16.72) |
| Serum albumin (g/dl) | 2.75 (2.30–3.00) |
| Serum procalcitonin (ng/ml) | 1.57 (0.53–29.56) |
| Serum lactate (mmol/L) | 9.80 (3.65–13.33) |
| eGFR (ml/min/1.73m2) | 54.50 (21.50–78.75) |
| Survival | 5 (31.3) |
Values are presented as median (interquartile range) or number (%).
APACHE: Acute Physiology and Chronic Health Evaluation; SAPS: Simplified Acute Physiology Score; CRRT: continuous renal replacement therapy; CRP: C-reactive protein; eGFR: estimated glomerular filtration rate.
a Shock: use of inotropic agent or vasopressor to maintain adequate tissue perfusion (mean arterial pressure over 65 mm Hg).
| Sample point |
aPTT (sec) |
P-value | |
|---|---|---|---|
| ECMO | Patient (n=16) | ||
| Baseline | 75.15 (57.80–120.00) | 69.70 (52.18–120.00) | 0.182a |
| S1 | 83.25 (49.55–120.00) | 82.80 (54.48–112.40) | 0.547b |
| S2 | 67.80 (48.28–98.05) | 63.95 (51.38–93.35) | 0.207b |
| S3 | 65.50 (56.30–104.20) | 61.00 (47.60–80.30) | 0.014b |
| S4 | 90.50 (59.28–115.20) | 70.70 (52.25–79.70) | 0.008b |
| S5 | 67.20 (59.95–102.60) | 70.30 (44.30–103.20) | 0.485a |
| S6 | 72.80 (61.85–95.00) | 65.70 (48.40–94.10) | 0.237b |
| S7 | 74.20 (60.65–87.85) | 67.50 (52.35–94.20) | 0.209a |
| S8 | 72.90 (57.30–90.00) | 63.55 (57.48–94.88) | 0.652b |
| S9 | 74.40 (56.03–95.98) | 61.70 (52.40–78.00) | 0.097b |
| S10 | 68.60 (60.13–84.43) | 61.10 (46.80–85.80) | 0.196b |
| S11 | 69.55 (59.20–80.75) | 61.35 (50.73–86.63) | 0.785b |
| S12 | 78.00 (59.70–80.40) | 66.05 (57.48–69.43) | 0.032b |
| S13 | 69.90 (61.05–89.15) | 72.70 (56.05–104.80) | 0.593b |
| S14 | 70.10 (53.65–86.10) | 64.70 (49.70–72.05) | 0.135b |
| S15 | 63.20 (58.25–102.30) | 61.00 (52.35–67.35) | 0.093a |
| Median | 79.25 (65.00–93.64) | 73.57 (54.78–86.66) | 0.010b |
Values are presented as median (interquartile range). Shapiro-Wilk’s test was employed for test of normality assumption.
aPTT: activated partial thromboplastin; VA-ECMO: veno-arterial extracorporeal membrane oxygenation, NM: nafamostat mesilate; S: sample point.
a Wilcoxon's signed-rank test;
b Paired t-test.
Shapiro-Wilk’s test was employed for test of normality assumption.
| Sample point |
aPTT (sec): heparin |
aPTT (sec): NM |
||||
|---|---|---|---|---|---|---|
| ECMO | Patient (n=7) | P-value | ECMO | Patient (n=7) | P-value | |
| Baseline | 60.10 (56.55–120.00) | 56.40 (50.95–120.00) | 0.068a | 66.60 (51.10–95.20) | 56.50 (46.05–69.70) | 0.066a |
| S1 | 66.80 (59.70–103.10) | 63.90 (50.70–86.20) | 0.223b | 66.50 (45.15–120.00) | 61.80 (46.45–94.10) | 0.401a |
| S2 | 77.50 (59.90–111.40) | 78.80 (57.40–107.70) | 0.757b | 62.40 (49.85–95.70) | 58.80 (51.85–93.10) | 0.477a |
| S3 | 67.90 (55.65–112.60) | 68.10 (57.80–100.75) | 0.782b | 64.80 (60.08–105.05) | 59.35 (48.40–72.18) | 0.071b |
| S4 | 73.60 (64.48–95.40) | 59.60 (49.30–87.95) | 0.103b | 60.95 (58.43–118.30) | 63.25 (48.75–77.00) | 0.117b |
| S5 | 70.00 (55.75–86.85) | 73.30 (52.90–110.80) | 0.238b | 70.10 (59.93–109.55) | 69.20 (42.85–88.58) | 0.385b |
| S6 | 67.95 (62.98–86.38) | 72.20 (55.30–96.60) | 0.440b | 70.40 (63.13–90.65) | 54.95 (48.05–71.78) | 0.004b |
| S7 | 70.60 (48.00–120.00) | 72.10 (47.30–120.00) | 0.271b | 76.00 (61.48–109.55) | 65.00 (50.10–87.60) | 0.036b |
| S8 | 84.05 (58.78–120.00) | 81.50 (63.08–120.00) | 1.000 a | 71.40 (56.75–103.25) | 63.70 (57.10–117.20) | 0.810b |
| S9 | 58.20 (48.45–93.30) | 62.35 (51.30–95.15) | 0.492b | 72.35 (56.03–77.85) | 57.20 (52.40–78.00) | 0.345a |
| S10 | 73.20 (61.00–91.50) | 92.20 (59.80–111.20) | 0.244b | 68.60 (64.40–94.40) | 61.10 (44.80–87.40) | 0.377b |
| S11 | 71.10 (62.20–97.90) | 78.00 (63.20–106.00) | 0.683b | 72.35 (61.55–89.40) | 56.95 (49.78–120.00) | 0.605b |
| S12 | 80.50 (53.05–95.50) | 72.05 (53.95–93.90) | 0.564b | 79.30 (59.70–81.30) | 67.10 (57.48–73.90) | 0.081b |
| S13 | 118.00 (58.30–120.00) | 94.90 (63.28–120.00) | 0.195b | 69.90 (61.05–97.75) | 72.70 (56.05–96.80) | 0.255b |
| S14 | 120.00 (57.00–120.00) | 100.20 (58.40–120.00) | 0.465b | 70.10 (51.45–95.55) | 64.70 (49.70–72.05) | 0.271b |
| S15 | 63.70 (58.60–98.60) | 70.90 (65.30–120.00) | 0.698b | 63.20 (60.60–102.30) | 61.30 (52.35–93.65) | 0.240b |
| Median | 72.95 (66.50–89.78) | 72.84 (61.43–91.64) | 0.768b | 73.13 (65.92–94.06) | 68.42 (54.94–81.07) | 0.031b |
Values are presented as median (interquartile range). Shapiro-Wilk’s test was employed for test of normality assumption.
aPTT: activated partial thromboplastin; VA-ECMO: veno-arterial extracorporeal membrane oxygenation; NM: nafamostat mesilate; S: sample point.
a Wilcoxon's signed-rank test;
b Paired t-test.
- 1. Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J 2017;63:60-7.ArticlePubMed
- 2. Buchtele N, Staudinger T, Schäfer AK, Bögl MS, Schoergenhofer C, Schwameis M. Anticoagulation in critically ill adults during extracorporeal circulation. Hamostaseologie 2021;41:294-306.ArticlePubMed
- 3. Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg 2014;118:731-43.PubMed
- 4. Olson SR, Murphree CR, Zonies D, Meyer AD, Mccarty OJ, Deloughery TG, et al. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J 2021;67:290-6.ArticlePubMedPMC
- 5. Protti A, Iapichino GE, Di Nardo M, Panigada M, Gattinoni L. Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation: a worldwide survey. Anesthesiology 2020;132:562-70.PubMed
- 6. Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015;29:90-101.ArticlePubMed
- 7. Selleng S, Selleng K. Heparin-induced thrombocytopenia in cardiac surgery and critically ill patients. Thromb Haemost 2016;116:843-51.ArticlePubMed
- 8. Akizawa T, Koshikawa S, Ota K, Kazama M, Mimura N, Hirasawa Y. Nafamostat mesilate: a regional anticoagulant for hemodialysis in patients at high risk for bleeding. Nephron 1993;64:376-81.ArticlePubMed
- 9. Sadahiro T, Yuzawa H, Kimura T, Oguchi M, Morito T, Mizushima S, et al. Current practices in acute blood purification therapy in Japan and topics for further study. Contrib Nephrol 2018;196:209-14.ArticlePubMed
- 10. Makino S, Egi M, Kita H, Miyatake Y, Kubota K, Mizobuchi S. Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy. Int J Artif Organs 2016;39:16-21.ArticlePubMed
- 11. Choi JY, Kang YJ, Jang HM, Jung HY, Cho JH, Park SH, et al. Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial. Medicine (Baltimore) 2015;94:e2392.ArticlePubMedPMC
- 12. Kumar G, Maskey A. Anticoagulation in ECMO patients: an overview. Indian J Thorac Cardiovasc Surg 2021;37(Suppl 2):241-7.ArticlePubMed
- 13. Buscher H, Vukomanovic A, Benzimra M, Okada K, Nair P. Blood and anticoagulation management in extracorporeal membrane oxygenation for surgical and nonsurgical patients: a single-center retrospective review. J Cardiothorac Vasc Anesth 2017;31:869-75.ArticlePubMed
- 14. Cho HJ, Kim DW, Kim GS, Jeong IS. Anticoagulation therapy during extracorporeal membrane oxygenator support in pediatric patients. Chonnam Med J 2017;53:110-7.ArticlePubMedPMC
- 15. Raiten JM, Wong ZZ, Spelde A, Littlejohn JE, Augoustides JG, Gutsche JT. Anticoagulation and transfusion therapy in patients requiring extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2017;31:1051-9.ArticlePubMed
- 16. Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artif Organs 1999;23:979-83.ArticlePubMed
- 17. Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 2009;13:154-75.ArticlePubMed
- 18. Aoyama T, Ino Y, Ozeki M, Oda M, Sato T, Koshiyama Y, et al. Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn J Pharmacol 1984;35:203-27.ArticlePubMed
- 19. Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY. Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs 2013;36:208-16.ArticlePubMed
- 20. Uchiba M, Okajima K, Abe H, Okabe H, Takatsuki K. Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity. Thromb Res 1994;74:155-61.ArticlePubMed
- 21. Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis 1985;15:164-8.ArticlePubMed
- 22. Baek NN, Jang HR, Huh W, Kim YG, Kim DJ, Oh HY, et al. The role of nafamostat mesylate in continuous renal replacement therapy among patients at high risk of bleeding. Ren Fail 2012;34:279-85.ArticlePubMed
- 23. Maruyama Y, Yoshida H, Uchino S, Yokoyama K, Yamamoto H, Takinami M, et al. Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: a three-year retrospective cohort study. Int J Artif Organs 2011;34:571-6.ArticlePubMed
- 24. Han W, San Bok J, Cho HJ, Yu JH, Na MH, Kang S, et al. Single-center experience of extracorporeal membrane oxygenation mainly anticoagulated with nafamostat mesilate. J Thorac Dis 2019;11:2861-7.ArticlePubMedPMC
- 25. Park JH, Her C, Min HK, Kim DK, Park SH, Jang HJ. Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation. Int J Artif Organs 2015;38:595-9.ArticlePubMed
- 26. Kikuchi M, Endo S, Inada K, Yamashita H, Takakuwa T, Nakae H, et al. Inhibitory effect of FUT-175 on the production of interleukin 8 and polymorphonuclear leukocyte elastase. Res Commun Mol Pathol Pharmacol 1995;87:269-74.PubMed
- 27. Yoshikawa T, Murakami M, Furukawa Y, Kato H, Takemura S, Kondo M. Effects of FUT-175, a new synthetic protease inhibitor on endotoxin-induced disseminated intravascular coagulation in rats. Haemostasis 1983;13:374-8.ArticlePubMed
- 28. Kamijo H, Mochizuki K, Nakamura Y, Mori K, Ichikawa M, Nitta K, et al. Nafamostat mesylate improved survival outcomes of sepsis patients who underwent blood purification: a nationwide registry study in Japan. J Clin Med 2020;9:2629. ArticlePubMedPMC
References
Figure & Data
References
Citations

- Approach to Decompensated Right Heart Failure in the Acute Setting
Catherine V. Levitt, Caitlin A. Williams, Jalil Ahari, Ali Pourmand
Journal of Clinical Medicine.2024; 13(3): 869. CrossRef - Critical Care Management of Severe Asthma Exacerbations
Shameek Gayen, Stephen Dachert, Bilal Lashari, Matthew Gordon, Parag Desai, Gerard Criner, Juan Cardet, Kartik Shenoy
Journal of Clinical Medicine.2024; 13(3): 859. CrossRef - Complications and Outcomes in 39,864 Patients Receiving Standard Care Plus Mechanical Circulatory Support or Standard Care Alone for Infarct-Associated Cardiogenic Shock
Jan-Sören Padberg, Jannik Feld, Leonie Padberg, Jeanette Köppe, Lena Makowski, Joachim Gerß, Patrik Dröge, Thomas Ruhnke, Christian Günster, Stefan Andreas Lange, Holger Reinecke
Journal of Clinical Medicine.2024; 13(4): 1167. CrossRef - Extra-Corporeal Membrane Oxygenation in Pregnancy
Tatsiana Romenskaya, Yaroslava Longhitano, Aman Mahajan, Gabriele Savioli, Antonio Voza, Manfredi Tesauro, Christian Zanza
Journal of Clinical Medicine.2024; 13(6): 1634. CrossRef - Anticoagulants in adult extracorporeal membrane oxygenation: alternatives to standardized anticoagulation with unfractionated heparin
Shu Tang, Liqing Xu, Hui Li, Zhanshen Wu, Qiang Wen
European Journal of Clinical Pharmacology.2023; 79(12): 1583. CrossRef - Management of cardiopulmonary bypass in patients with ischemic and hemorrhagic strokes in surgery for active infective endocarditis
Takahiro Yamazato, Hiroshi Munakata, Yutaka Okita
Indian Journal of Thoracic and Cardiovascular Surgery.2023;[Epub] CrossRef
- Figure
- We recommend
- Related articles
-
- An unusual case of relapsing arrhythmia during veno-arterial extracorporeal membrane oxygenation cannulation
- Plasma biomarkers for brain injury in extracorporeal membrane oxygenation
- Risk factors for cannula-associated arterial thrombosis following extracorporeal membrane oxygenation support: a retrospective study
- Association of pulmonary arterial pressure with volume status in patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation

 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite