Abstract
-
Background
- The inflammatory response that occurs following cardiac arrest can determine the long-term prognosis of patients who survive out-of-hospital cardiac arrest. We evaluated the correlation between C-reactive protein-to-albumin ratio (CAR) following cardiac arrest and long-term mortality.
-
Methods
- The current retrospective observational study examined patients with post-cardiac arrest syndrome (PCAS) treated with targeted temperature management at a single tertiary care hospital. We measured CAR at four time points (at admission and then 24 hours, 48 hours, and 72 hours after) following cardiac arrest. The primary outcome was the patients’ 6-month mortality. We performed multivariable and area under the receiver operating characteristic curve (AUC) analyses to investigate the relationship between CAR and 6-month mortality.
-
Results
- Among the 115 patients, 52 (44.1%) died within 6 months. In the multivariable analysis, CAR at 48 hours (odds ratio [OR], 1.130; 95% confidence interval [CI], 1.027–1.244) and 72 hours (OR, 1.241; 95% CI, 1.059–1.455) after cardiac arrest was independently associated with 6-month mortality. The AUCs of CAR at admission and 24, 48, and 72 hours after cardiac arrest for predicting 6-month mortality were 0.583 (95% CI, 0.489–0.673), 0.622 (95% CI, 0.528–0.710), 0.706 (95% CI, 0.615–0.786), and 0.762 (95% CI, 0.675–0.835), respectively.
-
Conclusions
- CAR at 72 hours after cardiac arrest was an independent predictor for long-term mortality in patients with PCAS.
-
Keywords: albumin; C-reactive protein; cardiac arrest; mortality; prognosis
INTRODUCTION
Post-cardiac arrest syndrome (PCAS) is still often fatal due to complications such as multiorgan failure or neurological damage [1,2]. This outcome is thought to be due to the damage caused by the systemic inflammation that occurs during the whole-body ischemic response that takes place in cardiac arrest. Tissue reperfusion injury, which is defined as an ischemia-reperfusion injury after the return of spontaneous circulation (ROSC), exacerbates the tissue damage [3].
The systemic inflammatory response can be evaluated using various laboratory markers. Several studies showing the statistical significance between laboratory markers and long-term mortality after out-of-hospital cardiac arrest (OHCA) have been conducted [4-8]. The neutrophil-to-lymphocyte ratio is a representative laboratory marker that has fair performance in predicting long-term mortality and neurological outcomes after OHCA [4]. One previous study revealed that the peak procalcitonin level at 24–48 hours after cardiac arrest can help predict neurological outcomes. The usefulness of these inflammatory markers has primarily focused on values within 48 hours [5]. In addition, C-reactive protein (CRP) levels, which are normally low, increase after ROSC, and many studies have examined their association with mortality and neurologic outcomes [6-8]. Conversely, various recent studies have reported that the CRP-to-albumin ratio (CAR) aided in determining the prognosis in cases of Guillain-Barré syndrome, traumatic brain injury, myocardial infarction, and stroke [9-12]. The patient’s body temperature is lowered during targeted temperature management (TTM), and the degree of inflammation decreases accordingly as reported by a study that maintained mild hypothermia for 72 hours [13]. The actual inflammatory response is considered to occur in the rewarming or post-rewarming period after TTM. Thus, the factors that can cause an inflammatory response after cardiac arrest are considered related after 48 hours.
We hypothesized that the mortality of patients with PCAS treated with TTM would be related to CAR beyond 48 hours after cardiac arrest. Therefore, we investigated the CAR at 72 hours after cardiac arrest to determine 6-month mortality and compared it with CARs at admission and 24 hours and 48 hours after cardiac arrest.
MATERIALS AND METHODS
Study Design and Population
The present study was retrospective and observational in design and included patients with PCAS treated with TTM at Chonnam National University Hospital between January 2018 and December 2020. We included patients with PCAS aged ≥18 years who were comatose following TTM. The exclusion criteria were as follows: patients who discontinued TTM due to transfer to other hospitals or passing away, those who underwent TTM with a temperature other than 33°C, those who needed support (such as continuous hemodialysis and/or percutaneous cardiopulmonary support during PCAS care), and those with missing data. This study was approved by the Institutional Review Board of Chonnam National University Hospital (No. CNUH-2021-141). The requirement of informed consent was waived due to the retrospective nature of the present study.
Targeted Temperature Management
We maintained the core body temperature of patients at 33°C for 24 hours. We continued to administer remifentanil and midazolam for sedation during TTM to enhance its efficiency and reduce the brain’s metabolism. We observed subclinical seizures in real time using amplitude-integrated electroencephalography.
Data Collection
Data related to the following parameters were obtained from the patients’ hospital records: age, sex, underlying disease, first on-scene monitored rhythm, time from sudden cardiac arrest to ROSC, cardiac arrest etiology, witnessed collapse, bystander cardiopulmonary resuscitation (CPR), and calculated the Sequential Organ Failure Assessment (SOFA) score within 24 hours of admission. In addition, serum laboratory results, such as lactate and glucose levels, artery blood gas analysis results (e.g., partial pressure of oxygen [PaO2] and partial pressure of carbon dioxide [PaCO2] were obtained within 24 hours after admission.
Blood samples for assessing albumin and CRP were taken at admission and again at 24, 48, and 72 hours after cardiac arrest. The high sensitivity nephelometric method (Dade Behring; Marburg, Germany) was used to measure CRP level, which was detected from 0.2 mg/L. CAR was obtained by dividing CRP level by albumin level. Albumin level was determined via enzymatic assay using an automatic analyzer (Hitachi-7600; Hitachi, Tokyo, Japan). We assessed 6-month mortality through telephone interviews with the patients or their caregivers. The primary outcome was 6-month mortality, whereas the secondary outcome was in-hospital mortality.
Statistical Analysis
We presented the categorical variables as frequencies and percentages, whereas continuous variables are shown as the mean±standard deviation or the median and interquartile range, depending on the Shapiro-Wilk test results. The categorical variables of the groups were comparatively analyzed using the chi-square test with a continuity correction in 2×2 tables. Continuous variables were compared between the groups using independent t-tests or Mann-Whitney U-tests. Repeated-measures analysis of variance was used to compare CRP level, albumin level, and CAR between survivors and non-survivors within 72 hours after cardiac arrest. Post-hoc analysis was performed using pairwise Mann-Whitney U-tests with a Bonferroni correction between survivor and non-survivor groups.
We performed a multivariable logistic regression analysis to identify the predictive force of CAR on 6-month or in-hospital mortality. Variables with P-values <0.20 on univariable comparisons were included in the multivariable regression model. We used a backward stepwise approach that sequentially eliminated variables with a threshold of P >0.10 to build a final adjusted regression model. Lastly, the presence of a shockable rhythm and bystander CPR were selected as adjusted variables (Supplementary Tables 1 and 2). CAR values at each time point were included in the final model. The results of the logistic regression analysis are presented as the odds ratio (OR) and 95% confidence interval (CI). We assessed the predictive performance of CAR to determine 6-month or in-hospital mortality using the area under the receiver operating characteristic (ROC) curve (AUC). The comparison of dependent ROC curves was performed using the method proposed by DeLong et al. [14]. All analyses were carried out using PASW version 18.0 (SPSS Inc., Chicago, IL, USA) and MedCalc version 19.0 (MedCalc Software, bvba, Ostend, Belgium). Statistical significance was set at P<0.05 (two-sided).
RESULTS
Patient Characteristics
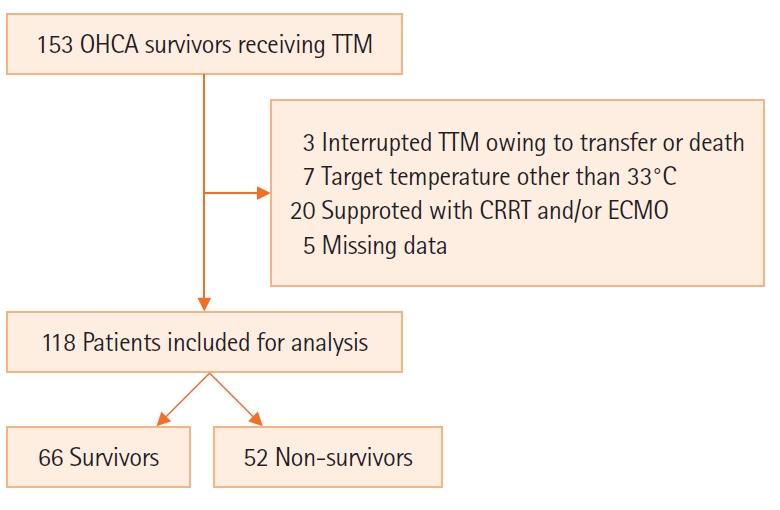
Among 115 cardiac arrest patients, 52 died within 6 months (44.1%) (Figure 1). The median age of the patients with OHCA was 58.7 years, and 91 male patients (77.1%) were included. In total, 81 collapses (68.6%) were witnessed by bystanders; 52 patients (44.1%) had a shockable rhythm, and the mean value of time from cardiac arrest to ROSC was 23.5 minutes (15.8–39.3 minutes).
Six-month mortality results indicated that non-survivors had a lower incidence of witnessed collapse and bystander CPR, as well as a higher incidence of a non-shockable rhythm and a noncardiac etiology. They also exhibited a prolonged time to ROSC compared with survivors. Non-survivors exhibited increased levels of lactate and PaCO2 after ROSC compared with survivors (Table 1). In-hospital mortality results indicated that non-survivors were older, had lower incidences of a shockable rhythm, and a more prolonged time to ROSC than survivors. Non-survivors exhibited an increased PaCO2 level following ROSC compared with survivors (Table 1).
CRP Level, Albumin Level, and CAR According to 6-Month or In-hospital Mortality
Six-month mortality results revealed that albumin levels at admission and 24, 48, and 72 hours after cardiac arrest were lower in non-survivors than in survivors. CRP level and CAR of non-survivors at 24, 48, and 72 hours after cardiac arrest were higher than those of survivors (Table 2). In-hospital mortality results revealed that albumin levels at admission and at 48and 72 hours after cardiac arrest were lower among non-survivors than among survivors. CRP level and CAR of non-survivors at 48and 72 hours after cardiac arrest were higher than those of survivors (Table 2).
CRP levels and CAR increased, and albumin levels decreased within 72 hours after cardiac arrest (Figure 2). Interactions between both 6-month mortality and in-hospital mortality and changes of CRP level and CAR over time were significant, but those for albumin level was not significant (Figure 2). Post-hoc analysis showed that albumin level was different at admission and at 48 and 72 hours after cardiac arrest between survivors and non-survivors irrespective of in-hospital and 6-month mortality (Figure 2). Post-hoc analysis showed that CRP level and CAR were different at 48 and 72 hours after cardiac arrest between survivors and non-survivors irrespective of in-hospital and 6-month mortality (Figure 2).
Prognostic Value of the CAR for 6-Month Mortality
After confounders were adjusted for, the CARs at 48 hours (OR, 1.291; 95% CI, 1.071–1.556) and 72 hours (OR, 1.515; 95% CI, 1.211–1.894) after cardiac arrest were independently associated with 6-month mortality (Table 3). Moreover, the CARs at 48 hours (OR, 1.181; 95% CI, 1.017–1.372) and 72 h (OR, 1.299; 95% CI, 1.101–1.533) after cardiac arrest were independently associated with in-hospital mortality (Table 3).
The AUCs of CAR at admission and 24, 48, and 72 hours after cardiac arrest for predicting 6-month mortality were 0.583 (95% CI, 0.489–0.673), 0.622 (95% CI, 0.528–0.710), 0.706 (95% CI, 0.615–0.786), and 0.762 (95% CI, 0.675–0.835), respectively (Table 4). Moreover, the AUCs of the CAR at admission and at 24, 48, and 72 hours after cardiac arrest for predicting in-hospital mortality were 0.576 (95% CI, 0.482–0.667), 0.595 (95% CI, 0.501–0.684), 0.687 (95% CI, 0.595–0.769), and 0.735 (95% CI, 0.645–0.812), respectively (Table 4). The AUC of the CAR 72 hours after cardiac arrest differed significantly from that at admission and at 24 hours after cardiac arrest but not from that observed 48 hours after cardiac arrest for predicting 6-month mortality or in-hospital mortality.
DISCUSSION
The primary finding in this study was the association between the CAR at 48 and 72 hours after cardiac arrest and 6-month mortality in the PCAS patient group. The CAR at 72 hours after cardiac arrest showed the highest performance for predicting 6-month mortality. Elevated levels of CRP were associated with 1-year mortality in acute cerebral infarction along with ischemic inflammatory response [15]. Systemic inflammation also occurs in patients with PCAS and ischemic injury. During cardiac arrest, the inflammatory response increases vascular permeability and destroys the blood-brain barrier (BBB), causing multiorgan ischemia, including in the brain [3,16]. CRP is an inflammatory biomarker that may be correlated with the severity of hypoxic brain damage following cardiac arrest. Engel et al. [5] reported that increased CRP levels after cardiac arrest are correlated with the SOFA scores of day 1 and three-month neurological outcomes. In another study, CRP at admission was associated with 30-day mortality in patients with PCAS [17]; however, CRP on admission was not associated with in-hospital or 6-month mortality in the present study. The reason for this difference may be the difference in the frequency of withdrawal of life-sustaining therapy (WLST). Unlike in Europe and the United States, WLST is rarely implemented for patients with PCAS in Korea. In a previous study [17], more patients had witnesses of their collapse (83.1% vs. 68.6%) and shockable rhythms (53.3% vs. 44.1%) compared with the present study. Thirty-day mortality (41.5%) in the previous study was higher than in-hospital mortality (33.9%) and similar to 6-month mortality (44.1%) in the present study. In one retrospective study, the increase in CRP levels in the TTM group was inhibited during cooling compared to the no-TTM group, and the difference gradually decreased after cooling [18]. In another retrospective study, the group with a poor neurologic outcome exhibited higher CRP levels at 48 and 72 hours than the group with a good neurologic outcome [19]. Thus, the inflammatory response is suppressed during TTM. After rewarming, the metabolism is restored, and the inflammatory response is activated, which results in increased CRP levels.
Hypoxia due to cardiac arrest causes increased the vascular permeability and impaired the BBB [20]. In an experimental study, hypoxic brain injury after cardiac arrest provoked BBB disruption and edema 24 hours after ROSC [21]. Increased vascular permeability leads to the loss of serum albumin, which results in reduced albumin levels following ischemic injury. In a previous study, albumin levels <3.5 g/dl were associated with in-hospital mortality and neurologic outcome [22]. In the present study, the albumin levels of non-survivors at each time point were usually <3.5 g/dl. Since high CRP levels and low albumin levels were associated with a poor prognosis in patients with PCAS, the CAR as calculated using CRP and albumin levels in the present study appears to reflect the severity of PCAS patients well.
CAR at admission is associated with in-hospital mortality of patients resuscitated from OHCA [23]. In this study [23], in-hospital mortality was 57.8% (59/102), which was higher than that reported by other studies, including our study [24,25]. In addition, data on TTM in previous studies have been lacking [23]. We postulated that TTM would delay the inflammatory response. Bisschops et al. [13] reported that mild hypothermia for 72 hours after cardiac arrest was correlated with a lowered inflammatory response. Several mechanisms are likely involved here. TTM contributes to a reduction in the white blood cell count and activity, which in turn prolongs the impairment of neutrophil function [13]. In an experimental study, inflammatory cytokines and gene expression were largely downregulated in the hypothermia-treated heart compared to the normothermic heart at 48 hours after TTM [26]. During in vivo experiments, neutrophil and monocyte chemotaxis, migration, phagocytosis, and oxidative metabolism were markedly decreased at 29°C compared with 37°C [27].
The present study has several limitations. First, it was retrospective in design and was conducted at a single center. Therefore, its results cannot be generalized immediately to the overall population. Additional prospective multicenter studies are needed to complement our research results. Second, other inflammatory markers (such as cytokines and chemokines) were not investigated in this study. Further studies that include these inflammatory markers will be needed in the future. Third, drugs such as vasopressors are generally used to improve cerebral perfusion after cardiac arrest and prevent secondary ischemic injury. In addition, norepinephrine increases the production of pro- and anti-inflammatory cytokines, but the effects of vasopressors (including norepinephrine) were not sufficiently considered in this study. Fourth, we did not investigate whether albumin was replaced according to albumin level or whether the replaced albumin affected the clinical outcomes of patients with PCAS.
In conclusion, the CAR 72 hours after cardiac arrest was related to 6-month mortality in the PCAS patient group and exhibited the best performance for predicting 6-month mortality. The CAR obtained 72 hours after cardiac arrest was an independent predictor of long-term mortality in the PCAS patient group.
KEY MESSAGES
▪ C-reactive protein-to-albumin ratio (CAR) is an effective marker of a systemic inflammatory response, including post-cardiac arrest syndrome (PCAS).
▪ Targeted temperature management can affect the systemic inflammatory response in the body by lowering the temperature.
▪ CAR more than 48 hours after cardiac arrest was an independent predictor for 6-month mortality in patients with PCAS.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
ACKNOWLEDGMENTS
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: JHL. Data curation: HHK, JHL. Formal analysis: DHL. Methodology: JHL, DHL. Project administration: JHL. Visualization: BKL. Writing–original draft: HHK, JHL. Writing–review & editing: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2022.00542.
Figure 1.Schematic diagram showing the number of patients in the present study. OHCA: out-of-hospital cardiac arrest; TTM: target temperature management; CRRT: continuous renal replacement therapy; ECMO: extracorporeal membrane oxygenation.
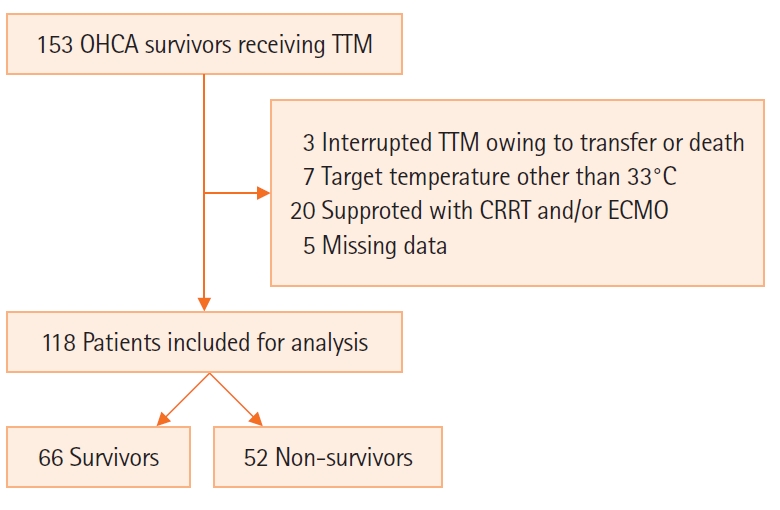
Figure 2.Repeated-measures analysis of variance of C-reactive protein (CRP), albumin, and C-reactive protein-to-albumin ratio (CAR) for 6-month mortality and in-hospital mortality 72 hours after cardiac arrest. (A) CRP level and 6-month mortality, (B) CRP level and in-hospital mortality, (C) albumin level and 6-month mortality, (D) albumin level and in-hospital mortality, (E) CAR and 6-month mortality, and (F) CAR and in-hospital mortality. a)P<0.013.

Table 1.Baseline characteristics by 6-month prognosis
|
Variable |
|
6-Month mortality
|
In-hospital mortality
|
|
Total (n=118) |
Survivor (n=66) |
Non-survivor (n=52) |
P-value |
Survivor (n=78) |
Non-survivor (n=40) |
P-value |
|
Demographics |
|
|
|
|
|
|
|
|
Age (yr) |
58.7±15.3 |
57.5 (47.8–67.0) |
63.5 (49.0–73.8) |
0.063 |
56.5±14.6 |
63.0±16.1 |
0.029 |
|
Male |
91 (77.1) |
53 (80.3) |
38 (73.1) |
0.480 |
61 (78.2) |
30 (75.0) |
0.872 |
|
Pre-existing illness |
|
|
|
|
|
|
|
|
Coronary artery disease |
18 (15.3) |
8 (12.1) |
10 (19.2) |
0.419 |
10 (12.8) |
8 (20.0) |
0.449 |
|
Hypertension |
45 (38.1) |
23 (34.8) |
22 (42.3) |
0.524 |
27 (34.6) |
18 (45.0) |
0.369 |
|
Diabetes |
33 (28.0) |
14 (21.2) |
19 (36.5) |
0.102 |
18 (23.1) |
15 (37.5) |
0.151 |
|
Renal impairment |
2 (1.7) |
1 (1.5) |
1 (1.9) |
1.000 |
2 (2.6) |
0 |
0.789 |
|
Cerebrovascular accident |
7 (5.9) |
2 (3.0) |
5 (9.6) |
0.267 |
4 (5.1) |
3 (7.5) |
0.917 |
|
Cardiac arrest characteristics |
|
|
|
|
|
|
|
|
Witnessed collapse |
81 (68.6) |
52 (78.8) |
29 (55.8) |
0.013 |
56 (71.8) |
25 (62.5) |
0.412 |
|
Bystander CPR |
79 (66.9) |
51 (77.3) |
28 (53.8) |
0.013 |
57 (73.1) |
22 (55.0) |
0.077 |
|
Shockable rhythm |
52 (44.1) |
43 (65.2) |
9 (17.3) |
<0.001 |
46 (59.0) |
6 (15.0) |
<0.001 |
|
Cardiac etiology |
81 (68.6) |
52 (78.8) |
29 (55.8) |
0.013 |
58 (74.4) |
23 (57.5) |
0.097 |
|
Time to ROSC (min) |
23.5 (15.8–39.3) |
20.0 (14.0–30.0) |
29.0 (20.5–44.8) |
0.002 |
20.0 (14.0–33.5) |
29.0 (22.0–44.8) |
0.002 |
|
Clinical characteristics after ROSC |
|
|
|
|
|
|
|
|
Lactate (mmol/L) |
7.0 (4.7–10.3) |
6.5 (4.1–9.3) |
8.6 (5.8–11.7) |
0.040 |
6.6 (4.2–9.4) |
8.8 (5.8–11.5) |
0.062 |
|
Glucose (mg/dl) |
262 (198–326) |
254 (198–307) |
277 (193–350) |
0.367 |
254 (191–308) |
288 (205–359) |
0.196 |
|
PaO2 (mm Hg) |
128 (89–211) |
116 (83–211) |
152 (97–222) |
0.102 |
116 (84–199) |
162 (97–238) |
0.099 |
|
PaCO2 (mm Hg) |
43 (33–60) |
37 (32–46) |
53 (35–68) |
0.002 |
38 (33–50) |
54 (35–68) |
0.011 |
|
SOFA score |
11 (9–12) |
10 (8–12) |
11 (9–12) |
0.104 |
10 (8–12) |
11 (9–12) |
0.063 |
Table 2.CRP level, albumin level, and CAR according to 6-Month mortality or in-hospital mortality
|
Variable |
|
6-month mortality
|
In-hospital mortality
|
|
Total (n=118) |
Survivor (n=66) |
Non-survivor (n=52) |
P-value |
Survivor (n=78) |
Non-survivor (n=40) |
P-value |
|
At admission |
|
|
|
|
|
|
|
|
CRP (mg/dl) |
0.2 (0.1–0.6) |
0.2 (0.0–0.4) |
0.2 (0.1–0.6) |
0.264 |
0.2 (0.0–0.5) |
0.2 (0.1–0.7) |
0.370 |
|
Albumin (g/dl) |
3.6 (3.3–3.9) |
3.7 (3.4–4.1) |
3.5 (3.1–3.8) |
0.002 |
3.7 (3.4–4.0) |
3.4 (3.0–3.6) |
<0.001 |
|
CAR |
0.0 (0.0–0.1) |
0.0 (0.0–0.1) |
0.1 (0.0–0.2) |
0.171 |
0.0 (0.0–0.1) |
0.1 (0.0–0.3) |
0.239 |
|
At 24 hours after CA |
|
|
|
|
|
|
|
|
CRP (mg/dl) |
6.1 (3.1–9.1) |
5.7 (2.3–7.8) |
6.7 (4.5–11.4) |
0.021 |
5.9 (2.8–8.4) |
6.7 (4.7–12.2) |
0.067 |
|
Albumin (g/dl) |
3.2 (2.9–3.5) |
3.3 (3.0–3.6) |
3.1 (2.8–3.5) |
0.020 |
3.3 (3.0–3.5) |
3.1 (2.8–3.5) |
0.051 |
|
CAR |
1.9 (0.9–3.0) |
1.7 (0.8–2.7) |
2.2 (1.3–3.6) |
0.024 |
1.8 (0.9–2.7) |
2.1 (1.3–3.8) |
0.094 |
|
At 48 hours after CA |
|
|
|
|
|
|
|
|
CRP (mg/dl) |
13.1 (8.7–18.6) |
10.8 (8.2–15.5) |
16.3 (12.2–21.8) |
<0.001 |
11.4 (8.4–16.3) |
16.8 (12.2–21.8) |
0.004 |
|
Albumin (g/dl) |
3.0 (2.7–3.3) |
3.2 (2.9–3.4) |
2.8 (2.6–3.1) |
<0.001 |
3.1 (2.8–3.4) |
2.8 (2.6–3.1) |
<0.001 |
|
CAR |
4.5 (2.9–6.5) |
3.6 (2.6–5.4) |
5.6 (3.7–8.1) |
<0.001 |
3.9 (2.6–5.6) |
5.9 (3.6–8.6) |
<0.001 |
|
At 72 hours after CA |
|
|
|
|
|
|
|
|
CRP (mg/dl) |
12.0 (8.2–17.5) |
9.4 (7.1–14.0) |
15.6 (11.3–25.0) |
<0.001 |
10.4 (7.3–15.3) |
15.8 (11.4–26.4) |
<0.001 |
|
Albumin (g/dl) |
3.0 (2.7–3.2) |
3.2 (2.9–3.3) |
2.7 (2.6–3.0) |
<0.001 |
3.1 (2.8–3.3) |
2.7 (2.5–3.1) |
<0.001 |
|
CAR |
4.1 (2.6–6.1) |
3.1 (2.3–4.7) |
5.6 (4.0–8.5) |
<0.001 |
3.4 (2.4–5.1) |
5.9 (4.0–9.1) |
<0.001 |
Table 3.Multivariable logistic regression analysis of the ability of the CAR to predict 6-month mortality or in-hospital mortality
|
Variable |
Adjusted OR (95% CI)a)
|
P-value |
|
6-Month mortality |
|
|
|
CAR at admission |
1.112 (0.865–1.429) |
0.408 |
|
CAR at 24 hours after cardiac arrest |
1.136 (0.918–1.405) |
0.242 |
|
CAR at 48 hours after cardiac arrest |
1.291 (1.071–1.556) |
0.007 |
|
CAR at 72 hours after cardiac arrest |
1.515 (1.211–1.894) |
<0.001 |
|
In-hospital mortality |
|
|
|
CAR at admission |
1.079 (0.873–1.334) |
0.482 |
|
CAR at 24 hours after cardiac arrest |
1.069 (0.896–1.276) |
0.458 |
|
CAR at 48 hours after cardiac arrest |
1.181 (1.017–1.372) |
0.029 |
|
CAR at 72 hours after cardiac arrest |
1.299 (1.101–1.533) |
0.002 |
Table 4.ROC analysis results of the CAR to predict 6-month mortality and in-hospital mortality
|
Variable |
Cutoff |
Sensitivity |
Specificity |
AUC |
P-value |
|
6-Month mortality |
|
|
|
|
|
|
CAR at admission |
>0.17 |
26.92 |
90.91 |
0.583 (0.489–0.673) |
0.116 |
|
CAR at 24 hours after cardiac arrest |
>1.79 |
65.38 |
57.58 |
0.622 (0.528–0.710) |
0.020 |
|
CAR at 48 hours after cardiac arrest |
>4.14 |
73.08 |
63.64 |
0.706 (0.615–0.786) |
<0.001 |
|
CAR at 72 hours after cardiac arrest |
>4.73 |
69.23 |
77.27 |
0.762 (0.675–0.835) |
<0.001 |
|
In-hospital mortality |
|
|
|
|
|
|
CAR at admission |
>0.17 |
30.00 |
89.74 |
0.576 (0.482–0.667) |
0.174 |
|
CAR at 24 hours after cardiac arrest |
>3.66 |
30.00 |
92.31 |
0.595 (0.501–0.684) |
0.097 |
|
CAR at 48 hours after cardiac arrest |
>5.70 |
52.50 |
78.21 |
0.687 (0.595–0.769) |
<0.001 |
|
CAR at 72 hours after cardiac arrest |
>5.90 |
50.00 |
87.18 |
0.735 (0.645–0.812) |
<0.001 |
References
- 1. Du L, Zheng K, Feng L, Cao Y, Niu Z, Song Z, et al. The first national survey on practices of neurological prognostication after cardiac arrest in China, still a lot to do. Int J Clin Pract 2021;75:e13759.ArticlePubMedPDF
- 2. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care 2015: section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015;95:202-22.ArticlePubMed
- 3. Widgerow AD. Ischemia-reperfusion injury: influencing the microcirculatory and cellular environment. Ann Plast Surg 2014;72:253-60.PubMed
- 4. Weiser C, Schwameis M, Sterz F, Herkner H, Lang IM, Schwarzinger I, et al. Mortality in patients resuscitated from out-of-hospital cardiac arrest based on automated blood cell count and neutrophil lymphocyte ratio at admission. Resuscitation 2017;116:49-55.ArticlePubMed
- 5. Engel H, Ben Hamouda N, Portmann K, Delodder F, Suys T, Feihl F, et al. Serum procalcitonin as a marker of post-cardiac arrest syndrome and long-term neurological recovery, but not of early-onset infections, in comatose post-anoxic patients treated with therapeutic hypothermia. Resuscitation 2013;84:776-81.ArticlePubMed
- 6. Mongardon N, Lemiale V, Perbet S, Dumas F, Legriel S, Guérin S, et al. Value of procalcitonin for diagnosis of early onset pneumonia in hypothermia-treated cardiac arrest patients. Intensive Care Med 2010;36:92-9.ArticlePubMedPDF
- 7. Mongardon N, Perbet S, Lemiale V, Dumas F, Poupet H, Charpentier J, et al. Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med 2011;39:1359-64.ArticlePubMed
- 8. Perbet S, Mongardon N, Dumas F, Bruel C, Lemiale V, Mourvillier B, et al. Early-onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med 2011;184:1048-54.ArticlePubMed
- 9. Ning P, Yang B, Yang X, Huang H, Shen Q, Zhao Q, et al. Clinical value of C-reactive protein/albumin ratio in Guillain-Barré syndrome. Neurol Sci 2021;42:3275-83.ArticlePubMedPDF
- 10. Wang R, He M, Ou X, Xie X, Kang Y. CRP albumin ratio is positively associated with poor outcome in patients with traumatic brain injury. Clin Neurol Neurosurg 2020;195:106051. ArticlePubMed
- 11. Karabağ Y, Çağdaş M, Rencuzogullari I, Karakoyun S, Artaç İ, İliş D, et al. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest 2018;48:e12928.ArticlePubMedPDF
- 12. Kocatürk M, Kocatürk Ö. Assessment of relationship between C-reactive protein to albumin ratio and 90-day mortality in patients with acute ischaemic stroke. Neurol Neurochir Pol 2019;53:205-11.PubMed
- 13. Bisschops LL, van der Hoeven JG, Mollnes TE, Hoedemaekers CW. Seventy-two hours of mild hypothermia after cardiac arrest is associated with a lowered inflammatory response during rewarming in a prospective observational study. Crit Care 2014;18:546. ArticlePubMedPMCPDF
- 14. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45.ArticlePubMed
- 15. Li YM, Liu XY. Serum levels of procalcitonin and high sensitivity C-reactive protein are associated with long-term mortality in acute ischemic stroke. J Neurol Sci 2015;352:68-73.ArticlePubMed
- 16. Amnuaypattanapon K, Thanachartwet V, Desakorn V, Chamnanchanunt S, Pukrittayakamee S, Sahassananda D, et al. Predictive model of return of spontaneous circulation among patients with out-of-hospital cardiac arrest in Thailand: the WATCH-CPR score. Int J Clin Pract 2020;74:e13502.ArticlePubMedPDF
- 17. Schriefl C, Schoergenhofer C, Poppe M, Clodi C, Mueller M, Ettl F, et al. Admission C-reactive protein concentrations are associated with unfavourable neurological outcome after out-of-hospital cardiac arrest. Sci Rep 2021;11:10279. ArticlePubMedPMCPDF
- 18. Dufner MC, Andre F, Stiepak J, Zelniker T, Chorianopoulos E, Preusch M, et al. Therapeutic hypothermia impacts leukocyte kinetics after cardiac arrest. Cardiovasc Diagn Ther 2016;6:199-207.ArticlePubMedPMC
- 19. Annborn M, Dankiewicz J, Erlinge D, Hertel S, Rundgren M, Smith JG, et al. Procalcitonin after cardiac arrest: an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation 2013;84:782-7.ArticlePubMed
- 20. Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci 2004;9:777-85.ArticlePubMed
- 21. Li J, Li C, Yuan W, Wu J, Li J, Li Z, et al. Mild hypothermia alleviates brain oedema and blood-brain barrier disruption by attenuating tight junction and adherens junction breakdown in a swine model of cardiopulmonary resuscitation. PLoS One 2017;12:e0174596.ArticlePubMedPMC
- 22. Yoon H, Song KJ, Shin SD, Ro YS, Hong KJ, Park JH. Effect of serum albumin level on hospital outcomes in out-of-hospital cardiac arrest. Hong Kong J Emerg Med 2020;27:293-9.ArticlePDF
- 23. Bingol Tanriverdi T, Patmano G, Bozkurt FT, Kaya BC, Tercan M. Prognostic value of C-reactive protein to albumin ratio in patients resuscitated from out-of-hospital cardiac arrest. Int J Clin Pract 2021;75:e14227.ArticlePubMedPDF
- 24. Akin M, Sieweke JT, Zauner F, Garcheva V, Tongers J, Napp LC, et al. Mortality in patients with out-of-hospital cardiac arrest undergoing a standardized protocol including therapeutic hypothermia and routine coronary angiography: experience from the HACORE Registry. JACC Cardiovasc Interv 2018;11:1811-20.PubMed
- 25. Tabi M, Burstein BJ, Ahmed A, Dezfulian C, Kashani KB, Jentzer JC. Shock severity and hospital mortality in out of hospital cardiac arrest patients treated with targeted temperature management. Shock 2021;55:48-54.ArticlePubMed
- 26. Shi J, Dai W, Kloner RA. Therapeutic hypothermia reduces the inflammatory response following ischemia/reperfusion injury in rat hearts. Ther Hypothermia Temp Manag 2017;7:162-70.ArticlePubMed
- 27. Wenisch C, Narzt E, Sessler DI, Parschalk B, Lenhardt R, Kurz A, et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth Analg 1996;82:810-6.ArticlePubMed
Citations
Citations to this article as recorded by

- Inflammatory response after out‐of‐hospital cardiac arrest—Impact on outcome and organ failure development
Asser M. J. Seppä, Markus B. Skrifvars, Pirkka T. Pekkarinen
Acta Anaesthesiologica Scandinavica.2023; 67(9): 1273. CrossRef - Comparison of Prognostic Performance between Procalcitonin and Procalcitonin-to-Albumin Ratio in Post Cardiac Arrest Syndrome
Ju Hee Yoon, Woo Sung Choi, Yong Su Lim, Jae Ho Jang
Journal of Clinical Medicine.2023; 12(14): 4568. CrossRef - C-reactive protein-to-albumin ratio as a biomarker in patients with sepsis: a novel LASSO-COX based prognostic nomogram
Xin Zhou, Shouzhi Fu, Yisi Wu, Zhenhui Guo, Wankang Dian, Huibin Sun, Youxia Liao
Scientific Reports.2023;[Epub] CrossRef
 , Ji Ho Lee1
, Ji Ho Lee1 , Dong Hun Lee1
, Dong Hun Lee1 , Byung Kook Lee2
, Byung Kook Lee2






 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite