Articles
- Page Path
- HOME > Acute Crit Care > Forthcoming articles > Article
-
Case Report
Neurosurgery Use of droxidopa for blood pressure augmentation after acute spinal cord injury: case reports -
Christopher S. Hong1
 , Muhammad K. Effendi2
, Muhammad K. Effendi2 , Abdalla A. Ammar3
, Abdalla A. Ammar3 , Kent A. Owusu3
, Kent A. Owusu3 , Mahmoud A. Ammar3
, Mahmoud A. Ammar3 , Andrew B. Koo1, Layton A. Lamsam1
, Andrew B. Koo1, Layton A. Lamsam1 , Aladine A. Elsamadicy1
, Aladine A. Elsamadicy1 , Gregory A. Kuzmik4, Maxwell Laurans1
, Gregory A. Kuzmik4, Maxwell Laurans1 , Michael L. DiLuna1, Mark L. Landreneau5
, Michael L. DiLuna1, Mark L. Landreneau5 -
DOI: https://doi.org/10.4266/acc.2021.01662
Published online: December 7, 2022
1Department of Neurosurgery, Yale School of Medicine, New Haven, CT, USA
2Department of Pharmacy Practice and Administration, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ, USA
3Department of Pharmacy, Yale New Haven Health System, New Haven, CT, USA
4MidState Medical Center, Hartford HealthCare Medical Group, Meriden, CT, USA
5Department of Neurology, Yale School of Medicine, New Haven, CT, USA
- Corresponding author: Mark L. Landreneau Department of Neurology, Yale School of Medicine, 15 York st, New Haven, 06520, USA Tel: +1-203-688-2341 (ext. 3064), E-mail: mark.landreneau@yale.edu
Copyright © 2022 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,857 Views
- 98 Download
Abstract
- Hypotension secondary to autonomic dysfunction is a common complication of acute spinal cord injury (SCI) that may worsen neurologic outcomes. Midodrine, an enteral α-1 agonist, is often used to facilitate weaning intravenous (IV) vasopressors, but its use can be limited by reflex bradycardia. Alternative enteral agents to facilitate this wean in the acute post-SCI setting have not been described. Here, we describe novel application of droxidopa, an enteral precursor of norepinephrine that is approved to treat neurogenic orthostatic hypotension, in the acute post-SCI setting. We outline the clinical course of two patients who were intolerant of midodrine due to reflex bradycardia and were successfully managed with droxidopa as an alternative treatment strategy. The addition of droxidopa avoided pacemaker placement in one patient. As such, droxidopa may be a viable enteral therapy to treat hypotension in patients after acute SCI who are otherwise not tolerating midodrine in order to wean off IV vasopressors. This strategy may avoid pacemaker placement and facilitate shorter stays in the intensive care unit, particularly for patients who are stable but require continued intensive care unit admission for IV vasopressors, which can be cost ineffective and human resource depleting.
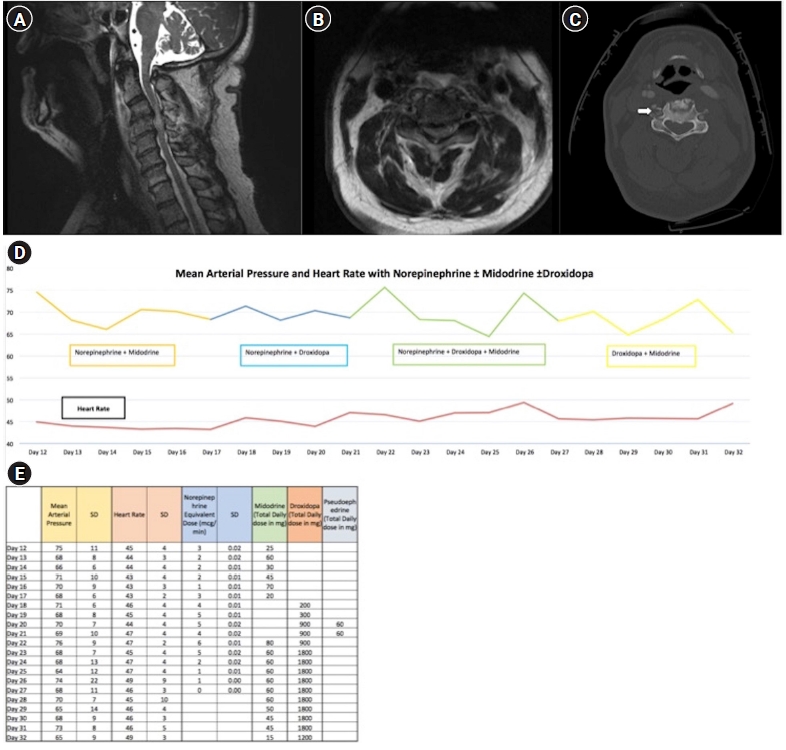
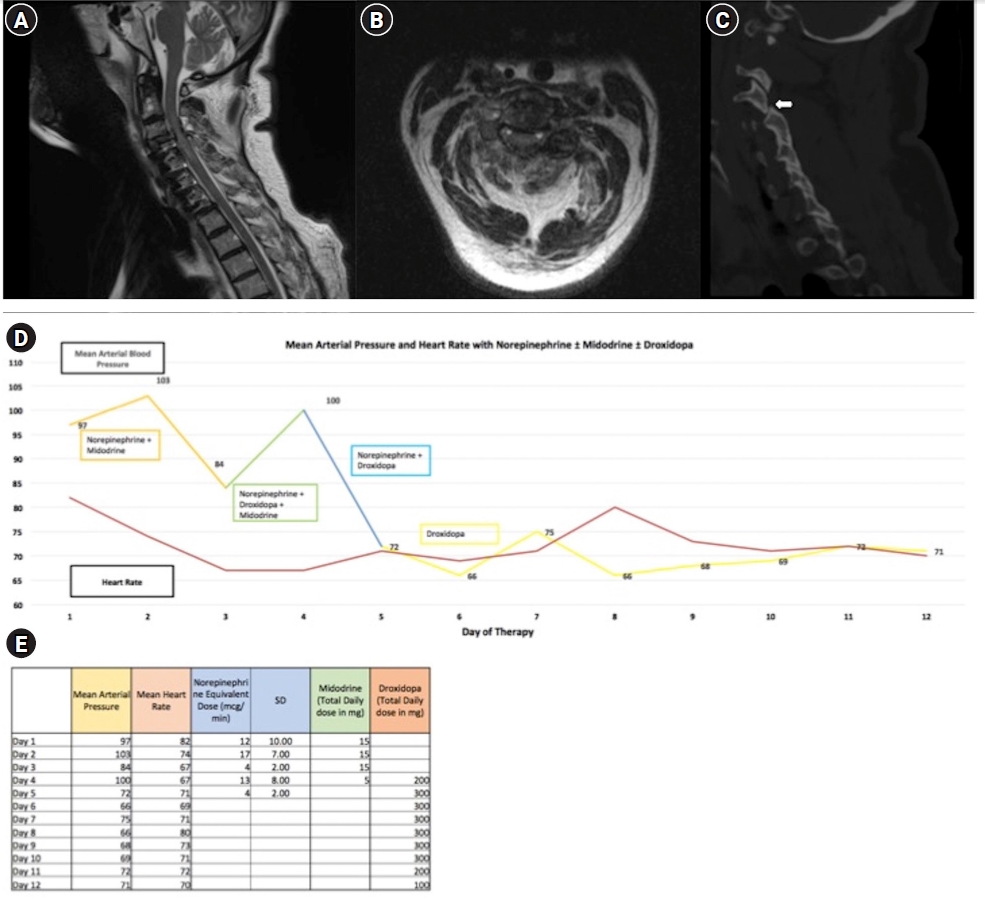
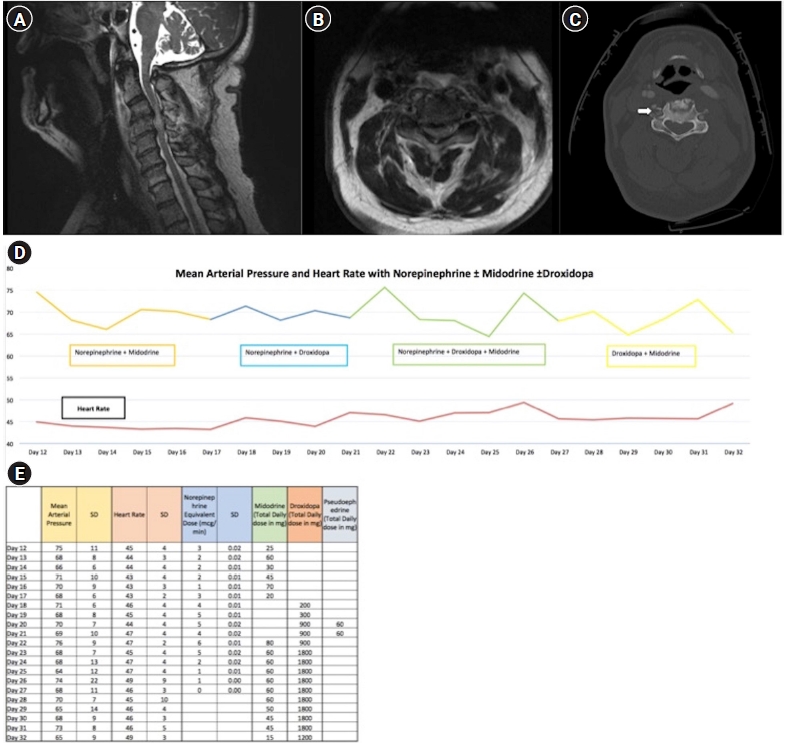
CASE REPORTS
DISCUSSION
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: CSH, MKE, AAA, MLL. Data curation: all authors. Formal analysis: CSH, MKE, AAA, MLL. Methodology: CSH, AAA, MLL. Project administration: CSH, MKE, AAA, MLL. Visualization: CSH, MKE, AAA, MLL. Writing–original draft: all authors. Writing–review & editing: all authors.
NOTES
Acknowledgments
- 1. Ryken TC, Hurlbert RJ, Hadley MN, Aarabi B, Dhall SS, Gelb DE, et al. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 2013;72 Suppl 2:84-92.ArticlePubMedPDF
- 2. Al-Abdouh A, Haddadin S, Matta A, Jabri A, Barbarawi M, Abusnina W, et al. Impact of adjuvant use of midodrine to intravenous vasopressors: a systematic review and meta-analysis. Crit Care Res Pract 2021;2021:5588483. ArticlePubMedPMC
- 3. Keating GM. Droxidopa: a review of its use in symptomatic neurogenic orthostatic hypotension. Drugs 2015;75:197-206.ArticlePubMedPDF
- 4. Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013;60:82-91.PubMed
- 5. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883-948.ArticlePubMed
- 6. Rizvi MS, Trivedi V, Nasim F, Lin E, Kashyap R, Andrijasevic N, et al. Trends in use of midodrine in the ICU: a single-center retrospective case series. Crit Care Med 2018;46:e628-33.ArticlePubMed
- 7. Santer P, Anstey MH, Patrocínio MD, Wibrow B, Teja B, Shay D, et al. Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomized clinical trial. Intensive Care Med 2020;46:1884-93.ArticlePubMedPMCPDF
- 8. Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation 2003;108:724-8.ArticlePubMed
- 9. Wecht JM, Rosado-Rivera D, Weir JP, Ivan A, Yen C, Bauman WA. Hemodynamic effects of L-threo-3,4-dihydroxyphenylserine (Droxidopa) in hypotensive individuals with spinal cord injury. Arch Phys Med Rehabil 2013;94:2006-12.ArticlePubMed
- 10. Canosa-Hermida E, Mondelo-García C, Ferreiro-Velasco ME, Salvador-de la Barrera S, Montoto-Marqués A, Rodríguez-Sotillo A, et al. Refractory orthostatic hypotension in a patient with a spinal cord injury: treatment with droxidopa. J Spinal Cord Med 2018;41:115-8.ArticlePubMed
- 11. Kaufmann H, Freeman R, Biaggioni I, Low P, Pedder S, Hewitt LA, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology 2014;83:328-35.ArticlePubMedPMC
References
Figure & Data
References
Citations


 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite