Abstract
-
Background
- Baseline diaphragmatic dysfunction (DD) at the initiation of non-invasive ventilation (NIV) correlates positively with subsequent intubation. We investigated the utility of DD detected 2 hours after NIV initiation in estimating NIV failure in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients.
-
Methods
- In a prospective-cohort design, we enrolled 60 consecutive patients with AECOPD initiated on NIV at intensive care unit admission, and NIV failure events were noted. The DD was assessed at baseline (T1 timepoint) and 2 hours after initiating NIV (T2 timepoint). We defined DD as ultrasound-assessed change in diaphragmatic thickness (ΔTDI) <20% (predefined criteria [PC]) or its cut-off that predicts NIV failure (calculated criteria [CC]) at both timepoints. A predictive-regression analysis was reported.
-
Results
- In total, 32 patients developed NIV failure, nine within 2 hours of NIV and remaining in the next 6 days. The ∆TDI cut-off that predicted NIV failure (DD-CC) at T1 was ≤19.04% (area under the curve [AUC], 0.73; sensitivity, 50%; specificity, 85.71%; accuracy; 66.67%), while that at T2 was ≤35.3% (AUC, 0.75; sensitivity, 95.65%; specificity, 57.14%; accuracy, 74.51%, hazard ratio, 19.55). The NIV failure rate was 35.1% in those with normal diaphragmatic function by PC (T2) versus 5.9% by CC (T2). The odds ratio for NIV failure with DD criteria ≤35.3 and <20 at T2 was 29.33 and 4.61, while that for ≤19.04 and <20 at T1 was 6, respectively.
-
Conclusions
- The DD criterion of ≤35.3 (T2) had a better diagnostic profile compared to baseline and PC in prediction of NIV failure.
-
Keywords: chronic obstructive pulmonary disease; diaphragmatic dysfunction; diaphragmatic thickness; intubation; non-invasive ventilation
INTRODUCTION
Non-invasive ventilation (NIV) is a first-line therapy in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) intended to support exhausted respiratory muscles to allow early recovery. Patients presenting late usually develop severe acidosis requiring early intubation [1]. Still, NIV failure remains a dreaded possibility for those tolerating an initial NIV trial [2]. Though studies indicate a positive correlation between baseline diaphragmatic dysfunction (DD) and subsequent NIV failure, such patients may tolerate NIV well if diaphragmatic functions (DFs) recover with NIV support [3,4]. A continued/new-onset DD after NIV initiation may indicate an impending need for intubation.
Various ultrasonographic parameters have been investigated to detect DD in AECOPD [5]. Among these, change in diaphragmatic thickness (ΔTDI) during tidal volume is the most sensitive. Other parameters (diaphragm thickness/velocity/excursion) may incorrectly label diaphragm atrophy in a low-body-weight individual with a thin-healthy diaphragm or miss an acutely paralyzed diaphragm with normal thickness [6]. An ultrasound-assessed ΔTDI value <20% during unassisted spontaneous breathing reflects DD [4,6]. However, the literature is sparse on validated criteria after NIV initiation. We hypothesized that NIV aids in the recovery of DF in some patients with AECOPD; continued or new-onset DD measured at 2 hours after initiating NIV will better identify those with early need for intubation, compared to baseline DD. Our primary objective was to estimate the utility of DD detected 2 hours after initiating NIV in predicting subsequent NIV failure events in AECOPD patients. We defined DD using both predefined (ΔTDI <20%) and calculated criteria (ΔTDI cut-off value). We also estimated the association of baseline DD at admission with NIV failure events.
MATERIALS AND METHODS
Enrolled Population
After All India Institute of Medical Sciences Rishikesh ethical approval (IEC no. AlIMS/IEC/20/111) and obtaining written informed consent, patients aged >18 years of either sex requiring NIV for AECOPD at intensive care unit (ICU) admission between July 2020 and 2021 were included in this prospective, outcome-assessor-blinded, observational cohort trial. We followed all ethical principles for medical research involving human subjects as per the Helsinki Declaration of 2013. The criteria for initiating NIV included acidosis (PaCO2 ≥45 mm Hg and arterial pH <7.25) and severe dyspnea, hypoxia, clinical signs of respiratory fatigue, or labored breathing despite supplemental oxygen therapy. We excluded those with acute pulmonary edema, interstitial lung disease, neuromuscular disease, chest-wall deformity, known diaphragmatic palsy, hemodynamic instability, intracranial hypertension, pregnancy, or any contraindication to NIV.
Measurement of DD
At ICU admission, we obtained a detailed medical history, performed a clinical examination, and collected baseline lab samples of all enrolled patients per standard institutional protocols. Each patient underwent a baseline diaphragmatic ultrasound in a supine position under B-mode (LOGIC QE, GE Healthcare). A linear ultrasound probe (7–12.0 MHz in a sterile cover) was placed just below the costophrenic sinus and was directed medially, cephalad, and dorsally between an anterior and posterior axillary line to obtain the best orientation of the hemidiaphragm (left/right) at the zone of apposition. We identified the diaphragm in the image as a three-layered structure (depth of 1–3 cm) consisting of a relatively less-echogenic muscle layer between two echogenic lines indicating the peritoneum (deeper) and parietal pleura (superficial). Diaphragmatic thickness (Td) was measured at end-inspiration and expiration under M-mode (T1 timepoint). The measurements were repeated thrice, and the higher Td value was recorded.
All patients were initiated on NIV (Puritan Bennett 840 ventilator, Fisher & Paykel Healthcare) via a non-vented full face mask interface (Best fit-2, Curative Medical Devices) using BIPAP mode, and no sedative was allowed. The NIV settings included an inspiratory positive airway pressure of 10 cm H2O, expiratory positive airway pressure of 5 cm H2O, and 40% fractional inspiratory oxygen, adjusted further to obtain a tidal volume of 8–10 ml/kg, respiratory rate <30 breaths/min, and a target oxygen saturation of 88%–94%. NIV was continued as long as possible on "day 1," for at least sixteen hours on "day 2," and for twelve hours on "day 3." It was discontinued on "day 4" or later based on clinical judgment or need for invasive ventilation (NIV failure). Indications for initiating invasive ventilation included respiratory/cardiac arrest, hemodynamic instability, no response to IV fluids and vasoactive drugs, arrhythmia, diminished consciousness, psychomotor agitation, aspiration/vomiting, life-threatening hypoxia, or arterial pH <7.25 after 2 hours on NIV. Ultrasonographic measurements were recorded using a similar methodology (on the same side) after 2 hours on NIV (T2 timepoint) or at intubation if a patient failed NIV. All patients received treatment as per standard institutional protocols by a blinded physician and were followed for 30 days post-ICU admission or death, whichever occurred earlier.
The ΔTDI (%) (at both timepoints) was calculated as ([end-inspiration Td–end-expiration Td]/end-expiration Td)×100. The DD was defined using predefined criteria (ΔTDI value <20%) or calculated criteria (ΔTDI cut-off value that predicted subsequent NIV failure at both timepoints). The arterial blood gas (ABG) parameters were measured at T1 and T2 or at the treating physician's discretion. Other recorded parameters included patient demographics, comorbidities, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment Score (SOFA) at ICU admission, durations of NIV and invasive ventilation, and ICU/hospital length of stay.
Statistical Analysis
The sample size was calculated using the Open-Epi Collection of Epidemiologic Calculator 3.01 (Andrew G. Dean, Kevin M. Sullivan). We expected an 85% NIV failure rate in patients with AECOPD and DD and a 20% NIV failure rate in those with normal DF after 2 hours on NIV, as estimated from pilot observations (10 patients). Using the "Fleiss with CC" model with a 95% confidence interval (CI) and 80% power, we required 22 patients. To increase the study strength and compensate for those requiring intubation before 2 hours on NIV or any dropouts, we planned to recruit 60 patients. For statistical analysis, we used the IBM SPSS 23.0 (IBM Corp.). The normality of data was assessed by the Kolmogorov-Smirnov test. Results were summarized as mean (standard deviation) or number (%). We analyzed the strength of the association between DD (at T1/T2) and NIV failure by chi-square/Fisher's exact test, receiver operator characteristic curve, youden index (YI), binary logistic, log-rank, and cox-proportional hazard (PH) regression tests. A P-value<0.05 was considered significant.
RESULTS
Patient Characteristics
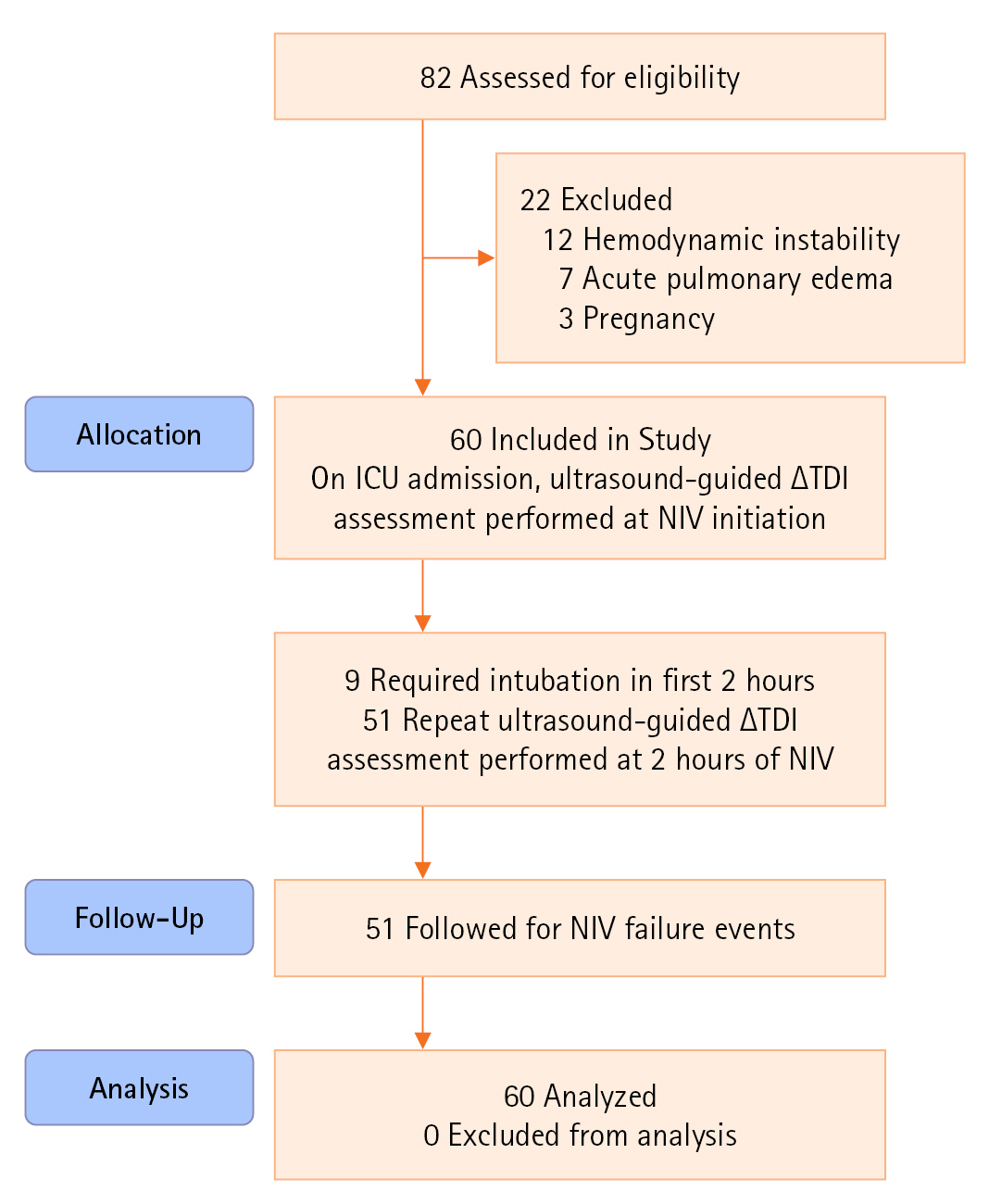
In total, 60 patients were included (Figure 1). The majority (67%) had comorbidities at admission, mainly diabetes (35%) and hypertension (28.3%). Baseline ABG parameters showed mixed respiratory acidosis (mean PaCO2, 64.42) and metabolic alkalosis/acidosis (mean pH, 7.20) with varying hypoxia. The baseline mean APACHE II and SOFA scores were 15.7 and 5.18, respectively (Table 1). The mean ∆TDI at T1 was 29.97% (n=60), while that at T2 was 30.05% (n=51); the mean ∆TDI of nine patients intubated before 2 hours was 14%.
Comparison between NIV Success and Failure
In total, 32 patients (53%) developed NIV failure, nine within 2 hours of NIV initiation (mean duration, 55.8 minutes) and the remaining 32 within the next 6 days. The mean time to intubation was 37.21 hours, while the durations of NIV and invasive ventilation were 2.58 and 4.63 days, respectively. Those with NIV failure were primarily males with a smoking history, diabetes, or cor pulmonale but had less frequent hypertension, lower baseline PaCO2/bicarbonate, lower ∆TDI at T1 and T2, shorter NIV duration, and extended hospital/ICU stay duration (Table 1).
DD Criteria to Predict NIV Failure
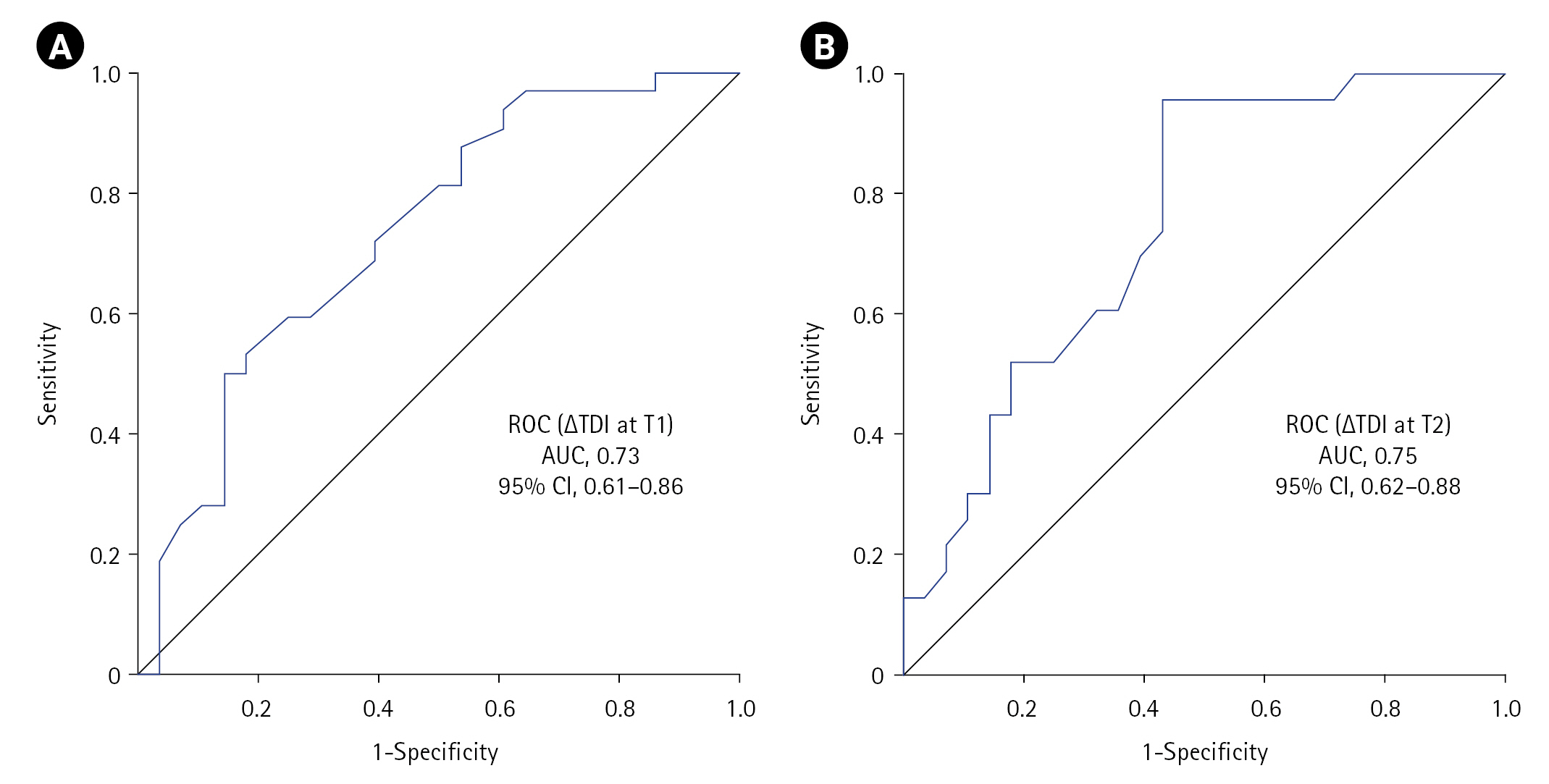
The ∆TDI cut-off that predicted subsequent NIV failure (DD calculated criteria) at T1 was ≤19.04% (sensitivity, 50%; specificity, 85.71%; YI, 35.71; accuracy, 66.67%) with an ROC area under the curve (AUC) of 0.73 (95% CI, 0.61–0.86; P=0.002), while that at T2 was ≤35.3% (sensitivity, 95.65%; specificity, 57.14%; YI, 52.80; accuracy, 74.51%) with an AUC of 0.75 (95% CI, 0.62–0.88; P=0.002) (Figure 2, Table 2). Twenty patients (n=60) experienced DD at T1 defined using either calculated (∆TDI ≤19.04%) or predefined criteria (∆TDI <20%), while 34 (continuous, 11; new-onset, 23) versus 14 (continuous, 11; new-onset, 3) had DD at T2 defined by calculated criteria (∆TDI ≤35.3%) or predefined criteria (∆TDI <20%) (n=51) (Table 3). A significant association was observed between DD and NIV failure at both timepoints, with the highest significance (P=0.002) achieved for the ≤35.3 criterion (Table 3). On univariate analysis, the odds ratio (OR) for NIV failure with DD criterion of ≤35.3 or <20 at T2 was 29.33 and 4.61, respectively, while that for ≤19.04 and <20 at T1 was 6, respectively. The ≤35.3 criteria had better sensitivity (95.65) and accuracy (74.51) but lower specificity (57.14) compared to that of ≤19.04 or <20 (85.71) (Table 2).
Covariates Predicting NIV Failure
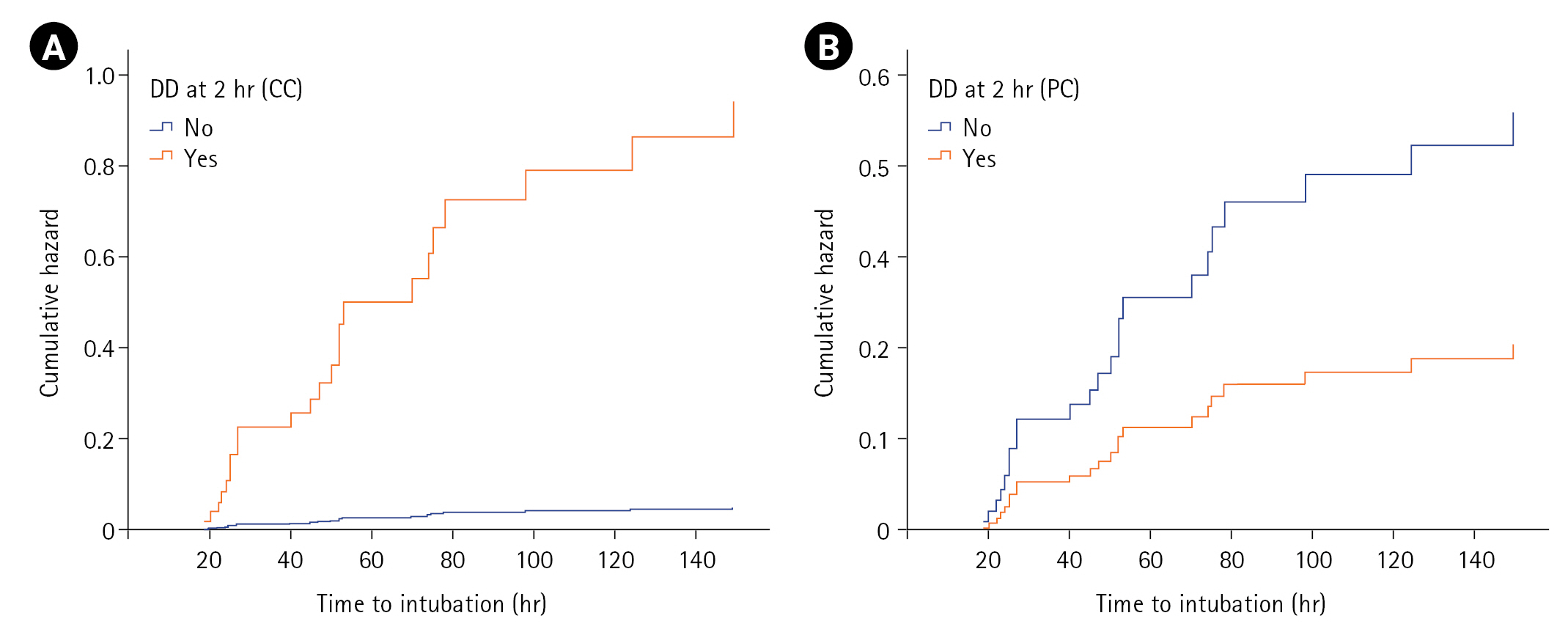
On log-rank/Cox-PH analysis of covariates at T1 predicting NIV failure (n=60), male sex (P=0.021), hypertension (P=0.031), heart rate (P=0.011), PaCO2 (P=0.041), ∆TDI (P=0.002), and number of patients with DD (P=0.001) achieved statistical significance, with DD having the highest hazard ratio (HR; 3.15) for NIV failure (T1) (Table 4). Variables of age, smoking history, comorbidities, steroid use, fever, APACHE II score, SOFA score, and other vitals/ABG parameters were not associated with NIV failure. However, multivariate Cox-PH analysis identified baseline heart rate (HR, 1.03; P=0.011) and ∆TDI at admission (HR, 0.96; P=0.004) as significant predictors. Though male sex attained an HR of 2.14, and hypertension had an HR of 0.39, these variables did not attain statistical significance. A similar log-rank/Cox-PH analysis at T2 identified male sex, heart rate (T1), ∆TDI (T2), and DD (T2) as significant predictors among 51 patients who tolerated NIV for the first 2 hours. On multivariable analysis at T2, including either DD by predefined or calculated criterion, parameters including male sex, baseline heart rate, and DD (calculated criteria) achieved statistical significance, with DD having the highest HR (19.55) for NIV failure (calculated criterion) (Table 4). Furthermore, the cumulative hazard for NIV failure was significantly higher for those with DD defined with calculated criteria (≤35.3) compared to predefined criteria (<20) (Figure 3).
DISCUSSION
We observed a 53% NIV failure rate in AECOPD patients initiated on NIV. The DD criterion of ≤35.3 (T2) had a better diagnostic profile and higher log odds and hazard ratio than other thresholds (<20; ≤19.04) in estimating NIV failure. Though the predefined criterion for DD (≤20) had better specificity, the calculated criterion (≤35.3) achieved higher sensitivity and better accuracy in predicting NIV failure.
Ultrasound-guided DF assessment is commonly performed at NIV initiation during unassisted spontaneous breathing in AECOPD [3-5,7]. An ultrasound-depicted ∆TDI value <20% at this timepoint indicates DD and risk of NIV failure. We observed a ∆TDI threshold of ≤19.04% for DD at T1, in agreement with the literature. Nine patients failed NIV within 2 hours of initiation; this was an expected outcome considering their lower baseline diaphragmatic reserve (mean ∆TDI, 17) compared to all patients (mean ∆TDI, 29.97). Thus, a baseline ∆TDI threshold ≤20% in this subset of patients successfully predicted impending NIV failure. Among the remaining 51 patients at 2 hours on NIV, one recovered off DD (∆TDI, 37), eleven had continuous DD (by either criterion), and three developed new-onset DD by predefined criterion and twenty developed new-onset DD by calculated criterion. Among patients with new-onset DD at T2, 14 in the calculated category versus two in the predefined category developed NIV failure. The NIV failure rate was 35.1% (T2) for those defined as having normal DF by a predefined criterion versus 5.9% (T2) for those defined as having normal DF by a calculated criterion. Thus, a cut off of <20% missed a significant proportion of patients who developed NIV failure; a ≤35.3% cut off, in opposition, missed only a single patient. This indicates that a higher ∆TDI threshold better estimates the DD after NIV initiation.
The DD criterion of ≤35.3 at T2 had a higher AUC (0.75) and showed a greater likelihood of NIV failure (OR, 29.33) than a threshold of ≤19.04 (AUC, 0.73; OR:,6). Cammarota et al. [7] also observed a higher AUC (0.98) for diaphragmatic excursion at one hour after initiating NIV compared to baseline values. Marchioni et al. [3] showed that AECOPD patients with DD at NIV initiation had a higher risk of NIV failure (HR, 6.2) compared to those with normal DF. While we could not identify studies evaluating ∆TDI after initiating NIV, similar thresholds were observed for ∆TDI as a predictor of successful weaning in ICU patients [8,9]. The univariate-Cox model estimated an HR of 17 by the DD criterion of ≤35.3, while an HR of 3.15 was estimated using other criteria (<20; ≤19.04) to predict NIV failure. The multivariate Cox-PH analysis indicated an even higher HR of 19.55 for the criterion of ≤35.3, while other criteria (<20; ≤19.04) did not attain statistical significance. The cumulative hazard of NIV failure was significant using a calculated criterion for DD (≤35.3).
The etiology of DD during AECOPD is multifactorial, involving both acute and chronic pathophysiological elements [10-12]. Though underlying chronic inflammation and steroid-induced diaphragmatic damage play a vital role, studies could not identify a comparable difference in ultrasound-assessed DF between those with stable COPD and healthy individuals [13]. This indicates some degree of diaphragmatic reserve in stable COPD that could not endure the increased workload and mechanical stress of AECOPD, leading to amplified functional diaphragmatic exhaustion/dysfunction during acute exacerbation. NIV is the first-line treatment intended to assist respiratory muscles, improve gaseous exchange, and thereby reduce the intubation rate in such patients [14,15]. Patients who respond to NIV have an improved pH within 1–4 hours after NIV initiation; hence, it is advisable to look for NIV failure as early as 2 hours after initiation [2,16,17]. We performed ∆TDI measurements 2 hours into NIV with this consideration. Moreover, some AECOPD patients with normal DF at ICU admission may develop NIV failure, while others with DD do not require intubation. This indicates the recovery/deterioration of DF after initiating NIV. Corbellini et al. [18] also reported an improvement in diaphragmatic mobility after in-patient pulmonary rehabilitation in AECOPD patients. Thus, repeat DF assessment after NIV initiation seems logical to identify those in need of intubation.
The significant Cox-covariates predicting NIV failure were male sex, hypertension, higher baseline heart rate, and hypercarbia. Studies have variably associated parameters of pH, hypercarbia, respiratory/heart rate, APACHE II score, and comorbidities with NIV failure [10,16]. An underlying difference in statistical interpretation (logistic vs. Cox regression) and sample size could contribute to such differences. Furthermore, inherent differences in unaccounted factors, including ethnicity, demographics, lifestyle, late hospital presentation, comorbidities, and organ reserve, could also account for such secondary outcomes. Still, a DD criterion of ≤35.3 showed adequate prediction of NIV failure.
Our study has some strengths. First, all studied timepoints were practically achievable and precisely analyzed in all included patients. Second, a skilled investigator with 5 years of ultrasound experience performed the sonographic measurements, and the outcome assessors were blinded to all other aspects; this allowed homogeneity in measurements and added to the authenticity of results. Our study has a few limitations. We performed ∆TDI estimation at two timepoints only. A serial time-bound analysis during the first 24 hours of NIV may better delineate DF changes and be an ideal review point for estimating NIV failure. Second, due to logistic issues, we could not evaluate the effect of lung hyperinflation during AECOPD. The relationship between lung volume change and ∆TDI after NIV initiation could better delineate the pathophysiological recovery and should be tested in future trials. Third, AECOPD could have a broad range of precipitating causes. An underlying difference in acute pulmonary pathophysiological/radiological involvement in different precipitants may also affect the underlying diaphragmatic reserve and possibly ∆TDI thresholds. To negate the effect of regional differences, we performed ∆TDI estimation on both hemidiaphragms and considered the best value for analysis.
In conclusion, a DD criterion of ≤35.3 at 2 hours after initiating NIV in AECOPD patients had a better diagnostic profile in predicting NIV failure compared to baseline or predefined criteria. Serial diaphragm evaluation and time-bound DD thresholds may better predict NIV failure, compared to single timepoint measurements and minimize intricacies related to delayed intubation.
KEY MESSAGES
▪ Though a positive correlation exists between baseline diaphragmatic dysfunction (DD) and subsequent non-invasive ventilation (NIV) failure, patients experience variable outcomes; thus, a dilemma remains on possible intubation after NIV.
▪ We observed that the DD criterion of ≤35.3 at 2 hours of NIV had a better diagnostic profile in predicting NIV failure compared to that of <20 in acute exacerbation of chronic obstructive pulmonary disease patients.
▪ Serial diaphragm evaluation and time-bound DD thresholds may better predict NIV failure and minimize the intricacies of delayed intubation.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: NBP, GJ. Data curation: NBP, UC, SC, HI. Formal analysis: GJ, ASB. Methodology: GJ, ASB. Project administration: GJ. Visualization: GJ. Writing–original draft: NBP, GJ. Writing–review & editing: GJ, UC, ASB, SC, HI.
Acknowledgments
None.
Figure 1.Strengthening the reporting of observational studies in epidemiology (STROBE) flow diagram of patients studied. ICU: intensive care unit; ∆TDI: change in diaphragmatic thickness; NIV: non-invasive ventilation.

Figure 2.Receiver operating characteristic (ROC) curve showing the utility of diaphragmatic dysfunction in predicting non-invasive ventilation (NIV) failure at (A) T1 and (B) T2 timepoints. ∆TDI: change in diaphragmatic thickness; T1: at NIV initiation; T2: at 2 hours into NIV; AUC: area under the curve; CI: confidence interval.
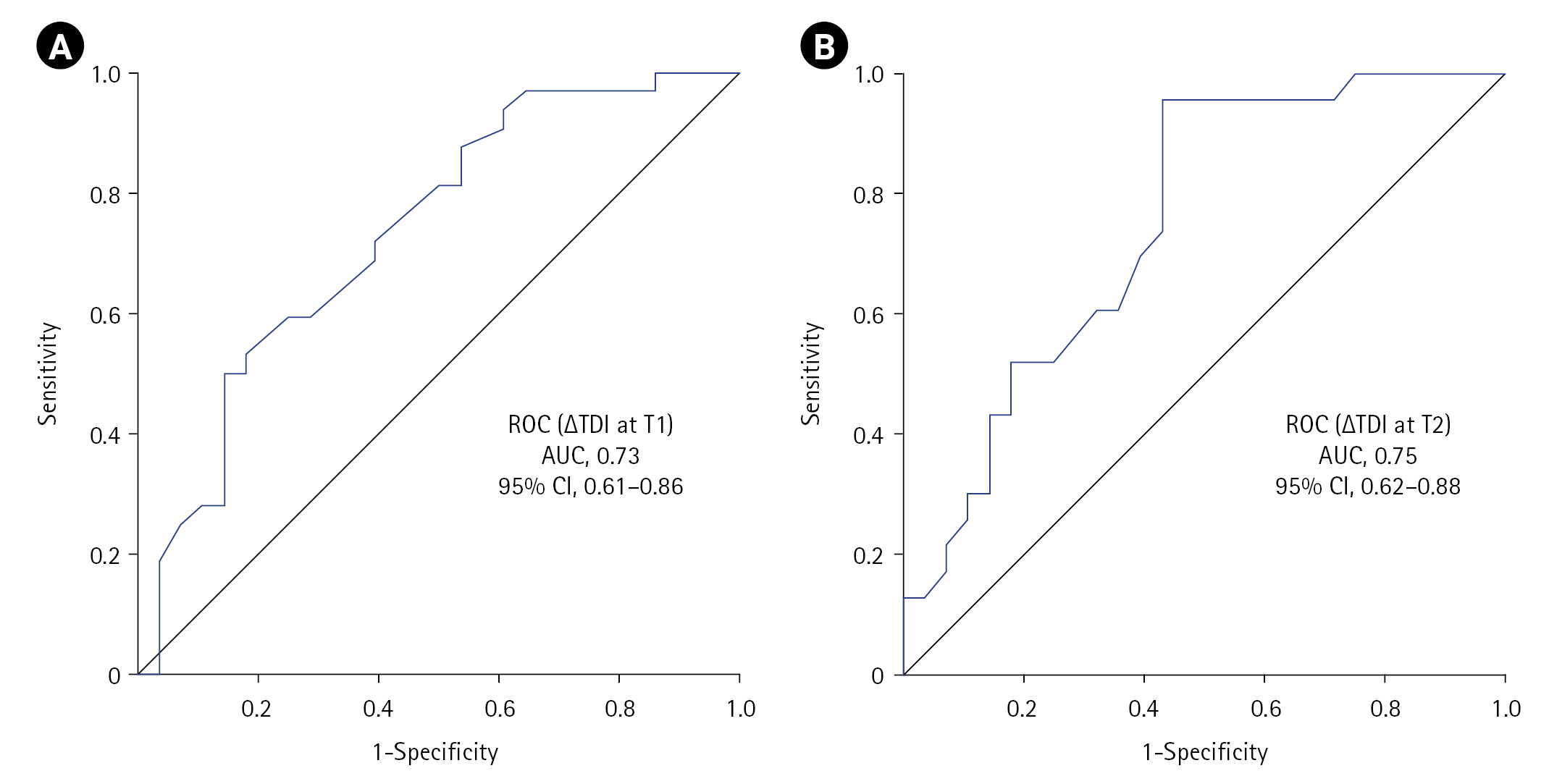
Figure 3.Cox-proportional hazards plot showing the cumulative hazard for intubation as predicted by diaphragmatic dysfunction (DD) at 2 hours of non-invasive ventilation using (A) calculated criterion (CC) and (B) predefined criterion (PC).
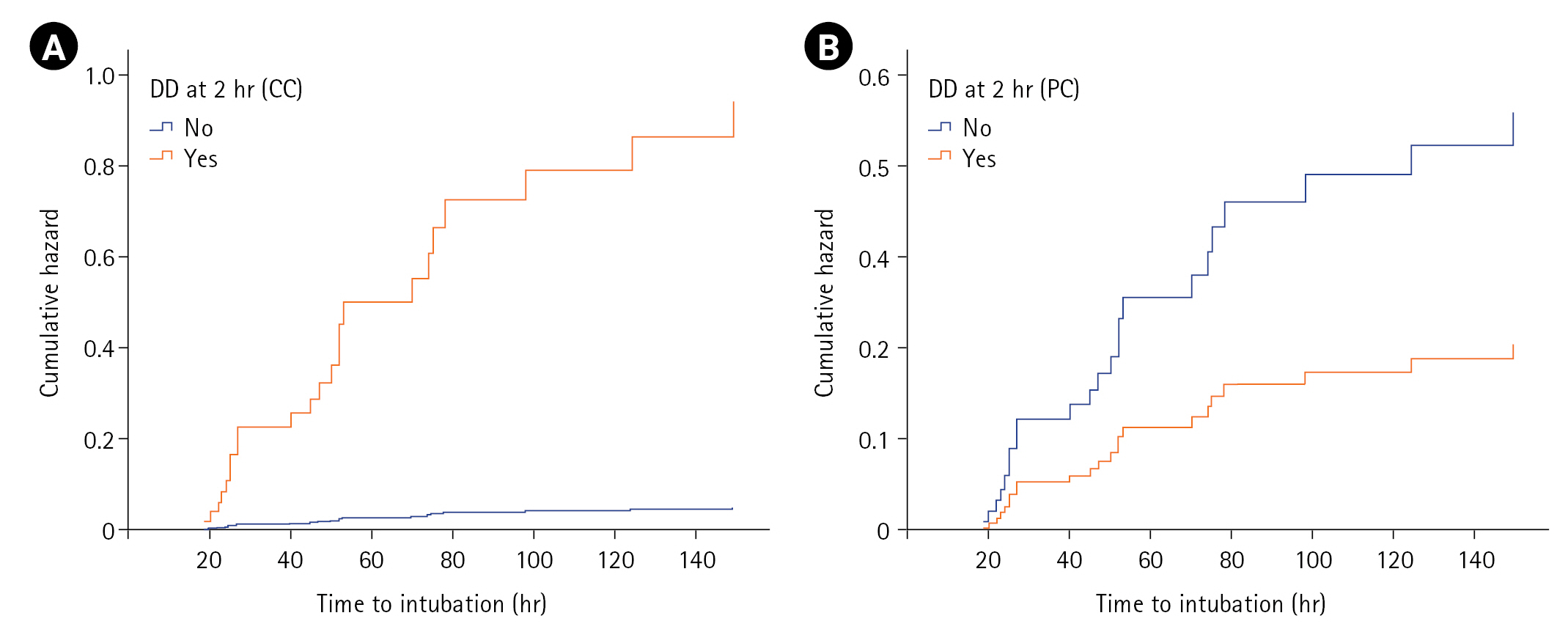
Table 1.Comparison of patients according to NIV outcome
|
Variable |
Total (n=60) |
NIV failure (n=32) |
NIV success (n=28) |
P-value |
|
Age (yr) |
59±11 |
58±12 |
60±11 |
0.514 |
|
Male |
36 (60) |
24 (67) |
12 (33) |
0.017 |
|
Smoking history |
22 (37) |
15 (68) |
7 (32) |
0.109 |
|
Comorbidity |
|
|
|
|
|
Diabetes |
21 (35) |
13 (62) |
8 (38) |
0.419 |
|
Hypertension |
17 (28) |
5 (29) |
12 (71) |
0.024 |
|
Cor pulmonale |
12 (20) |
8 (67) |
4 (33) |
0.349 |
|
Pneumonia |
12 (20) |
6 (50) |
6 (50) |
1.000 |
|
Steroid use |
29 (48) |
14 (48) |
15 (52) |
0.605 |
|
Fever |
17 (28) |
11 (65) |
6 (35) |
0.390 |
|
APACHE II score |
15.7±2.5 |
15.6±2.7 |
15.9±2.2 |
0.649 |
|
Baseline SOFA score |
5.2±2.0 |
5.0±2.1 |
5.4±1.8 |
0.444 |
|
Baseline ABG analysis |
|
|
|
|
|
pH |
7.20±0.08 |
7.20±0.07 |
7.20±0.09 |
0.920 |
|
Lactate |
1.8±0.8 |
1.9±0.8 |
1.8±0.7 |
0.632 |
|
PaO2
|
81.8±25.9 |
85.6±27.6 |
77.5±23.6 |
0.229 |
|
PaCO2
|
64.4±17.4 |
59.3±12.3 |
70.3±20.5 |
0.014 |
|
Bicarbonate |
25.1±9.3 |
22.8±5.2 |
27.8±12.1 |
0.046 |
|
∆TDI at T1 |
30.0±15.5 |
24.4±12.6 |
37.0±16.0 |
0.001 |
|
∆TDI at T2/intubation |
27.6±14.6 |
20.6±10.6 |
35.7±14.5 |
<0.001 |
|
before 2 hours |
|
Time to intubation (hr) (n=32) |
37.2±37.6 |
- |
- |
- |
|
Duration of NIV (day) |
2.6±1.9 |
1.6±1.5 |
3.8±1.6 |
<0.001 |
|
Duration of invasive ventilation (day) (n=32) |
4.6±6.2 |
- |
- |
- |
|
ICU stay (day) |
8.4±5.0 |
10.3±6.0 |
6.3±2.3 |
0.001 |
|
Hospital stay (day) |
10.5±5.5 |
12.1±6.8 |
8.6±2.5 |
0.011 |
Table 2.Predictive analysis of NIV failure with respect to DD at both timepoints
|
Parameter |
Criteria (∆TDI) |
SE (%) |
SP (%) |
Accuracy (%, 95% CI) |
OR (95% CI) |
P-value |
|
DD at T1 (PC) |
<20.00 |
50.00 |
85.71 |
66.67 (53.31–78.31) |
6 (1.69–21.26) |
0.006 |
|
DD at T1 (CC) |
≤19.04 |
50.00 |
85.71 |
66.67 (53.31–78.31) |
6 (1.69–21.26) |
0.006 |
|
DD at T2 (PC) |
<20.00 |
43.48 |
85.71 |
66.67 (52.08–79.24) |
4.61 (1.21–17.65) |
0.025 |
|
DD at T2 (CC) |
≤35.30 |
95.65 |
57.14 |
74.51 (60.37–85.67) |
29.33 (3.45–249.12) |
0.002 |
Table 3.Comparison of the association between DD and NIV failure at both timepoints
|
Parameter |
NIV failure |
P-value |
|
At T1 (PC/CC) |
|
0.006 |
|
DD (n=20) |
16 (80.0) |
|
|
No DD (n=40) |
16 (40.0) |
|
At T2 (PC) |
|
0.029 |
|
DD (n=14) |
10 (71.4) |
|
|
No DD (n=37) |
13 (35.1) |
|
At T2 (CC) |
|
<0.001 |
|
DD (n=34) |
22 (64.7) |
|
|
No DD (n=17) |
1 (5.9) |
|
DD at T2 (PC) |
|
0.066 |
|
None (n=37) |
13 (35.1) |
|
|
New (n=3) |
2 (66.7) |
|
Continuous (n=11) |
8 (72.7) |
|
DD at T2 (CC) |
|
<0.001 |
|
None (n=17) |
1 (5.9) |
|
|
New (n=23) |
14 (60.9) |
|
Continuous (n=11) |
8 (72.7) |
Table 4.Univariate and multivariable Cox proportional hazards regression analysis for all identified significant predictors of NIV failure
|
Variable |
Univariate |
Multivariate |
|
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
|
T1 |
|
|
|
|
|
|
|
Sex (male) |
2.57 |
1.15–5.73 |
0.021 |
2.14 |
0.95–4.95 |
0.067 |
|
Hypertension |
0.35 |
0.13–0.91 |
0.031 |
0.39 |
0.14–1.13 |
0.083 |
|
Baseline heart rate (bpm) |
1.03 |
1.0–1.05 |
0.011 |
1.03 |
1.01–1.06 |
0.011 |
|
Baseline PaCO2 (mm Hg) |
0.97 |
0.95–1.0 |
0.041 |
0.98 |
0.95–1.01 |
0.151 |
|
∆TDI at T1 |
0.96 |
0.94–0.98 |
0.002 |
0.96 |
0.92–1.01 |
0.004 |
|
DD at T1 (PC/CC) |
3.15 |
1.57–6.32 |
0.001 |
1.43 |
0.42–4.89 |
0.566 |
|
T2 |
|
|
|
|
|
|
|
Sex (male) |
2.62 |
1.03–6.66 |
0.043 |
2.88a)
|
1.08–7.63a)
|
0.034a)
|
|
2.84b)
|
1.05–7.66b)
|
0.039b)
|
|
Hypertension |
0.36 |
0.12–1.07 |
0.067 |
- |
- |
- |
|
Baseline heart rate (bpm) |
1.03 |
1.0–1.05 |
0.03 |
1.04a)
|
1.01–1.07a)
|
0.007a)
|
|
1.04b)
|
1.01–1.06b)
|
0.005b)
|
|
Baseline PaCO2 (mm Hg) |
0.97 |
0.95–1.0 |
0.07 |
- |
- |
- |
|
∆TDI at T2 |
0.96 |
0.93–0.98 |
0.007 |
0.99a)
|
0.95–1.04a)
|
0.876a)
|
|
0.92b)
|
0.85–0.99b)
|
0.040b)
|
|
DD at T2 (CC) |
17.0 |
2.28–126.53 |
0.006 |
19.55a)
|
2.08–84.07a)
|
0.009a)
|
|
DD at T2 (PC) |
2.52 |
1.10–5.76 |
0.029 |
0.45b)
|
0.07–2.74b)
|
0.385b)
|
References
- 1. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50:1602426. ArticlePubMed
- 2. Chen T, Bai L, Hu W, Han X, Duan J. Risk factors associated with late failure of noninvasive ventilation in patients with chronic obstructive pulmonary disease. Can Respir J 2020;2020:8885464. ArticlePubMedPMCPDF
- 3. Marchioni A, Castaniere I, Tonelli R, Fantini R, Fontana M, Tabbì L, et al. Ultrasound-assessed diaphragmatic impairment is a predictor of outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease undergoing noninvasive ventilation. Crit Care 2018;22:109. ArticlePubMedPMCPDF
- 4. Antenora F, Fantini R, Iattoni A, Castaniere I, Sdanganelli A, Livrieri F, et al. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: a pilot study. Respirology 2017;22:338-44.ArticlePubMedPDF
- 5. Laghi FA Jr, Saad M, Shaikh H. Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulm Med 2021;21:85. ArticlePubMedPMCPDF
- 6. Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013;47:319-29.ArticlePubMedPMCPDF
- 7. Cammarota G, Sguazzotti I, Zanoni M, Messina A, Colombo D, Vignazia GL, et al. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir Care 2019;64:1469-77.ArticlePubMed
- 8. Qian Z, Yang M, Li L, Chen Y. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: a systematic review and meta-analysis. BMJ Open 2018;8:e021189.ArticlePubMedPMC
- 9. Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest 2017;152:1140-50.ArticlePubMed
- 10. Steriade AT, Johari S, Sargarovschi N, Necula D, Tudose CE, Ionita D, et al. Predictors of outcome of noninvasive ventilation in severe COPD exacerbation. BMC Pulm Med 2019;19:131. ArticlePubMedPMCPDF
- 11. Haegens A, Schols AM, Gorissen SH, van Essen AL, Snepvangers F, Gray DA, et al. NF-κB activation and polyubiquitin conjugation are required for pulmonary inflammation-induced diaphragm atrophy. Am J Physiol Lung Cell Mol Physiol 2012;302:L103-10.ArticlePubMed
- 12. Troyer AD, Wilson TA. Action of the diaphragm on the rib cage. J Appl Physiol (1985) 2016;121:391-400.ArticlePubMed
- 13. Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:200-5.ArticlePubMedPMC
- 14. Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med 2014;174:1982-93.ArticlePubMedPMC
- 15. Shah NM, D’Cruz RF, Murphy PB. Update: non-invasive ventilation in chronic obstructive pulmonary disease. J Thorac Dis 2018;10(Suppl 1):S71-9.ArticlePubMedPMC
- 16. Duan J, Wang S, Liu P, Han X, Tian Y, Gao F, et al. Early prediction of noninvasive ventilation failure in COPD patients: derivation, internal validation, and external validation of a simple risk score. Ann Intensive Care 2019;9:108. ArticlePubMedPMCPDF
- 17. Lim SY, Lim G, Lee YJ, Cho YJ, Park JS, Yoon HI, et al. Ultrasound Assessment of diaphragmatic function during acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Int J Chron Obstruct Pulmon Dis 2019;14:2479-84.PubMedPMC
- 18. Corbellini C, Boussuges A, Villafañe JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care 2018;63:1271-80.ArticlePubMed
Citations
Citations to this article as recorded by

- Advancing healthcare through thoracic ultrasound research in older patients
Simone Scarlata, Chukwuma Okoye, Sonia Zotti, Fulvio Lauretani, Antonio Nouvenne, Nicoletta Cerundolo, Adriana Antonella Bruni, Monica Torrini, Alberto Finazzi, Tessa Mazzarone, Marco Lunian, Irene Zucchini, Lorenzo Maccioni, Daniela Guarino, Silvia Fabbr
Aging Clinical and Experimental Research.2023; 35(12): 2887. CrossRef
 , Gaurav Jain1
, Gaurav Jain1 , Udit Chauhan2
, Udit Chauhan2 , Ajeet Singh Bhadoria3
, Ajeet Singh Bhadoria3 , Saurabh Chandrakar1
, Saurabh Chandrakar1 , Haritha Indulekha1
, Haritha Indulekha1








 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite