Abstract
- The most feared complication of left ventricular thrombus (LVT) is the occurrence of systemic thromboembolic events, especially in the brain. Herein, we report a patient with severe sepsis who suffered recurrent devastating embolic stroke. Transthoracic echocardiography revealed apical ballooning of the left ventricle with a huge LVT, which had not been observed in chest computed tomography before the stroke. This case emphasizes the importance of serial cardiac evaluation in patients with stroke and severe medical illness.
-
Keywords: echocardiography; sepsis; stroke; thrombosis
Left ventricular regional or global wall akinesia results in blood stasis, and sometimes leads to left ventricular thrombus (LVT) formation. LVT is reported to complicate 5% of patients with acute myocardial infarction (AMI) and stress-induced cardiomyopathy (SICMP), and 11-44% of patients with end-stage dilated cardiomyopathy.[1] A feared and fatal complication of LVT is a systemic thromboembolic event, especially to the brain;[2] therefore, early detection and treatment is very important. Patients with AMI usually undergo routine echocardiography after the event, so it is relatively easier to discover an LVT, but in patients with SICMP, cardiac evaluation is sometimes delayed, making it harder to diagnose an LVT. Herein, we report a case of a well-visualized huge LVT associated with apical ballooning of the left ventricle in a patient with septic shock. The LVT caused repeated devastating embolic strokes; however, anticoagulation was not feasible due to combined intracranial bleeding.
Case Report
A 52-year-old woman with mental retardation presented with general weakness. She had a medical history of diabetes mellitus, hypertension, and left nephrectomy due to emphysematous pyelonephritis. A visiting caregiver detected that the patient had developed poor oral intake, dysphagia, myalgia, and general weakness, and brought her to the emergency room. Prior medication was unknown. On her initial examination, blood pressure was 118/65 mmHg, pulse rate 98 beats/min, breathing rate 18 breaths/min, and body temperature 36.5°C. Arterial blood gas levels in room air were pO2 93.5 mmHg and pCO2 33.0 mmHg, with pH 7.491. Leukocytosis and left-shift was present, with a white blood cell count of 18,880/mm3, segmented neutrophil fraction of 89.5%, and C-reactive protein level was elevated at 12.48 mg/dL. Anemia (hemoglobin 8.6 g/dL) was also identified. Kidney and liver function tests were normal. Initial chest X-ray showed right lower lung consolidation (Fig. 1A), and following chest computed tomography (CT) revealed right lower lung field pneumonia with lung abscess and pleural effusion (Fig. 1B). There was no significant cardiac abnormality in this chest CT imaging (Fig. 1B). Electrocardiogram was normal sinus rhythm with nonspecific T wave abnormality (Fig. 3A). Brain CT showed no acute lesions (Fig. 2A).
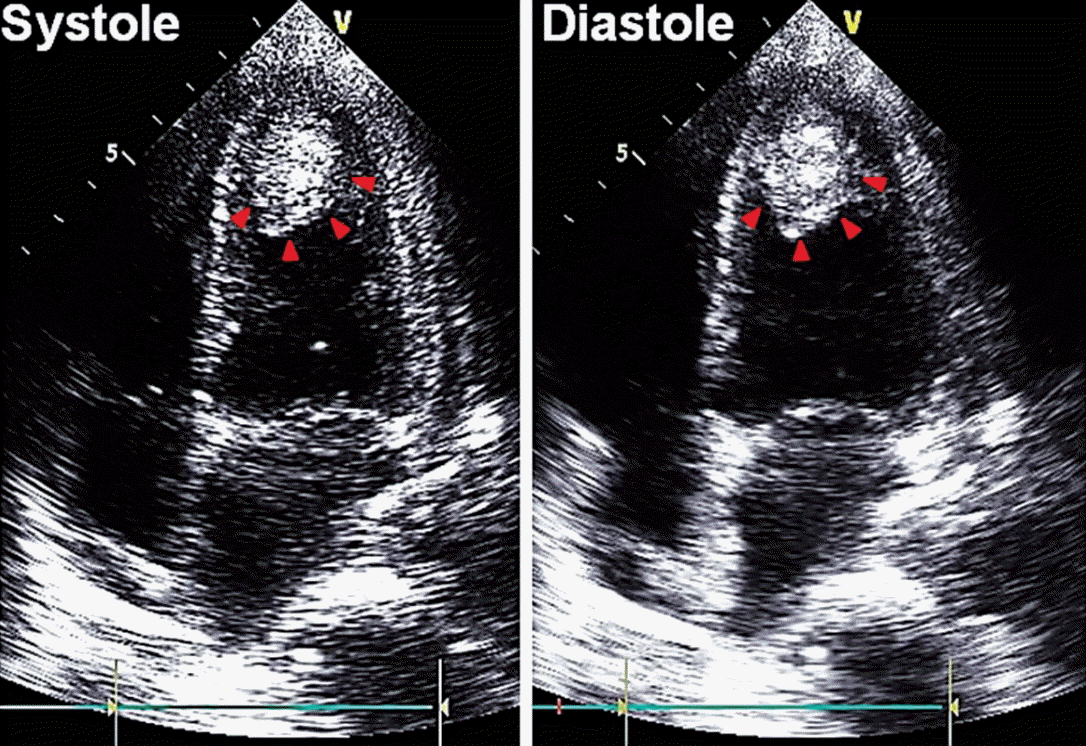
Under the diagnosis of complicated pneumonia, oxygen supplementation and intravenous antibiotics ceftriaxone and metronidazole were started. However, mental status and hypoxemia did not improve, and shock developed on the third day of hospitalization. She was transferred to the intensive care unit and mechanically ventilated. With antibiotics, inotropics and vasopressors, her vital signs stabilized. However, her mental status suddenly deteriorated to stupor on the fourth day of hospitalization, and brain CT and magnetic resonance imaging (MRI) were performed. Brain CT revealed large acute infarctions in bilateral frontoparietal and left temporal lobes (Fig. 2B), which was compatible with territories of bright signal intensity in diffusion-weighted brain MRI (Fig. 2C). Angiography by MRI showed total occlusion of bilateral proximal internal carotid arteries on MRI (Fig. 2D). Due to the patient’s poor general condition and little predicted surgical benefit, she was treated conservatively with medication including mannitolization. Twelve-lead electrocardiogram showed that T-waves were markedly inverted in all precordial and limb leads (Fig. 3B), and cardiac enzymes were elevated (peak CK-MB 8.9 ng/mL [normal range, < 6.6 ng/mL], peak cardiac troponin-I 12.75 ng/mL [normal range, < 0.028 ng/mL]). In order to evaluate cardiac problems, bed-side transthoracic echocardiography (TTE) was performed. TTE showed depressed left ventricular systolic function (ejection fraction 37%) with akinesia of the apical wall. Mid-portion and basal wall contraction of the left ventricle was relatively preserved. Notably, there was a highly mobile and huge mural thrombus (2.3 × 3.7 cm) at the left ventricular apex (Fig. 4). Anticoagulation was impossible due to coincidental multiple intracranial hemorrhages (Fig. 2E). Coronary angiogram was also deferred due to poor general condition and inability to use antiplatelets or anticoagulants. On the eighth day of hospitalization, brain CT follow-up revealed aggravated brain swelling and a new acute infarction in the right temporal lobe (Fig. 2F). Her pupils became fully dilated and fixed with impaired corneal reflexes. Once more, decompressive surgery was considered but rejected due to little operative benefit. On the 12th day of hospitalization, the patient’s mental status deteriorated to coma, and brain CT showed multiple intracranial hemorrhages with severe brain swelling and transtentorial herniation, and terminal supportive care was recommended.
Over time with conservative management, her vital signs stabilized, but she remained comatose. On the 28th day, she was transferred to another hospital for long term palliative care.
Discussion
This is a case of a 52-year-old woman who presented with pneumonia and septic shock, and then suffered recurrent embolic strokes. Although she was found to have a huge LVT by TTE, anticoagulation was impossible due to combined intracranial bleeding.
The pathophysiology underlying the patient’s apical akinesia and depressed left ventricle systolic function is uncertain. Although SICMP was highly suspected from the typical echocardiographic findings and clinical situation,[3-5] coronary obstruction could not be ruled out, because coronary angiogram was deferred due to little therapeutic benefit. Considering that myocardial dysfunction in severe sepsis usually manifests as global systolic and diastolic dysfunction of both the left and right side of the heart,[6] sepsis-related myocardial dysfunction is a less likely diagnosis in this patient. However, there are overlapping concepts between SICMP and myocardial depression in sepsis, with the common features being that both are caused by stress and are reversible. Also, the sepsis itself or the inotropic used during treatment can cause SICMP to develop in sepsis. [5,7] Besides, there are also arguments that sepsis-induced myocardial depression is actually SICMP incited by acute cardiac sympathetic disruption with adrenergic spill-over in the majority of cases.[8]
A devastating complication of this case was recurrent embolic strokes associated with a huge LVT. Embolic events occurred in 18% of patients with AMI and LVT, and a third of patients with SICMP and LVT.[1] The most frequent site of embolization is the brain (75%).[1] Embolic strokes in these situations are usually extensive, and in patients with severe systolic dysfunction, an embolic event is associated with a four-fold increase in mortality.[2] Earlier detection and management of LVT could contribute to a better prognosis for the patient.
Contrast-enhanced MRI is reported to have the highest sensitivity and specificity for LVT detection.[9] However, only a few tertiary clinical centers are ready to perform urgent MRI due to its costs and time. Echocardiography is the most commonly used technique to diagnose both myocardial dysfunction and associated LVT formation in the clinical field.[10] The portability of echocardiography is especially valuable when managing critically ill patients in the intensive care unit. Given that an LVT and thromboembolism may occur at any stage of myocardial infarction or SICMP, repeat echocardiography is also useful for the detection and follow-up of LVT.[1] In our case, cardiac morphology was normal and LVT was not visible in the initial chest CT. However, TTE, performed several days later following an embolic stroke, identified a large LVT with apical ballooning. Serial cardiac monitoring and evaluation should be considered to detect progressive structural changes of the heart in critically ill patients if hemodynamic and clinical parameters have changed.
Anticoagulation is effective, resolving LVT in most of the cases and preventing fatal embolic complications. Therefore, early anticoagulation is recommended in patients with suspected SICMP and low risk of bleeding, irrespective of the confirmed presence of LVT.[1] However, anticoagulation was not possible in our patient, despite the large LVT, because of the high risk of intracranial bleeding. Surgical removal was also infeasible due to the poor general condition of the patient.
In conclusion, we presented a patient with severe sepsis who had a huge LVT and left ventricle apical akinesia associated with recurrent embolic strokes. This case emphasizes the importance of cardiac evaluation in patients with embolic strokes and severe medical illnesses.
NOTES
-
No potential conflict of interest relevant to this article was reported.
Fig. 1.Chest radiographic examination at initial admission. Chest X-ray (A) showed right lung consolidation, which was identified as lung abscess in chest computed tomography (red arrow) (B). There was no abnormality of cardiac contour and no visible mural thrombus in the left ventricle (red arrowheads).
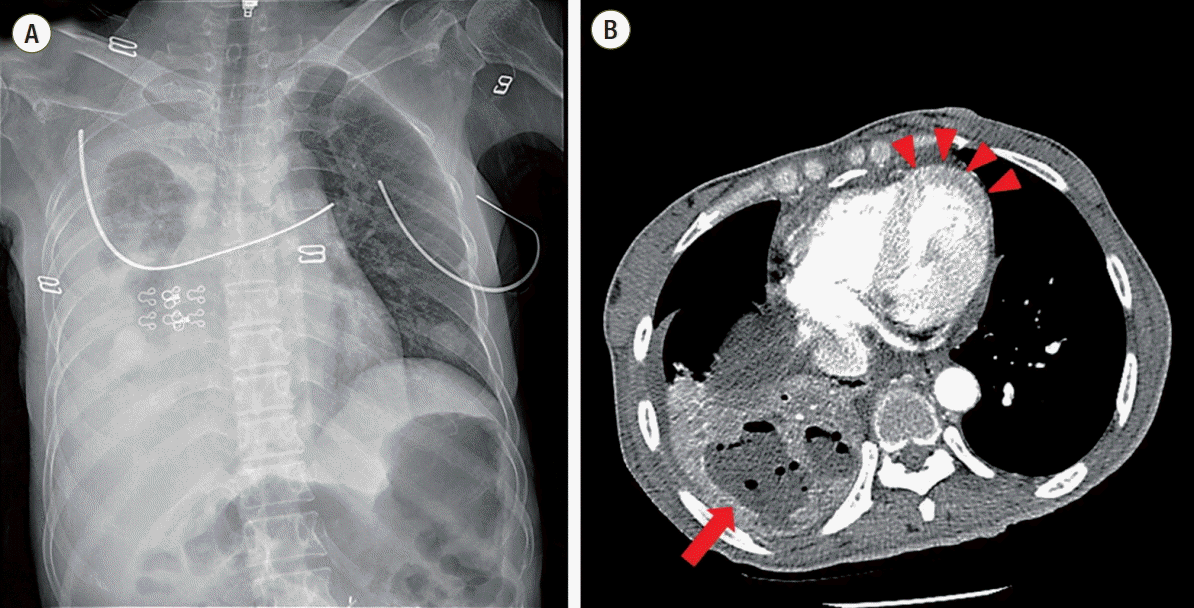
Fig. 2.The results of brain imaging studies. Normal brain computed tomographic findings at initial admission (A). Large multiple territorial infarctions were shown in brain computed tomography (hypo-attenuated lesions) (B) and diffusion-weighted magnetic resonance imaging (high signal intensities) (C) during stupor. There was total occlusion of the proximal portions of both internal carotid arteries (red arrows) (D). Combined brain stem and intraventricular hemorrhages (hyper-attenuated lesions) were shown (E). In serial examinations, a new acute infarction was seen in the right temporal lobe (red arrowheads) in brain computed tomography (F).
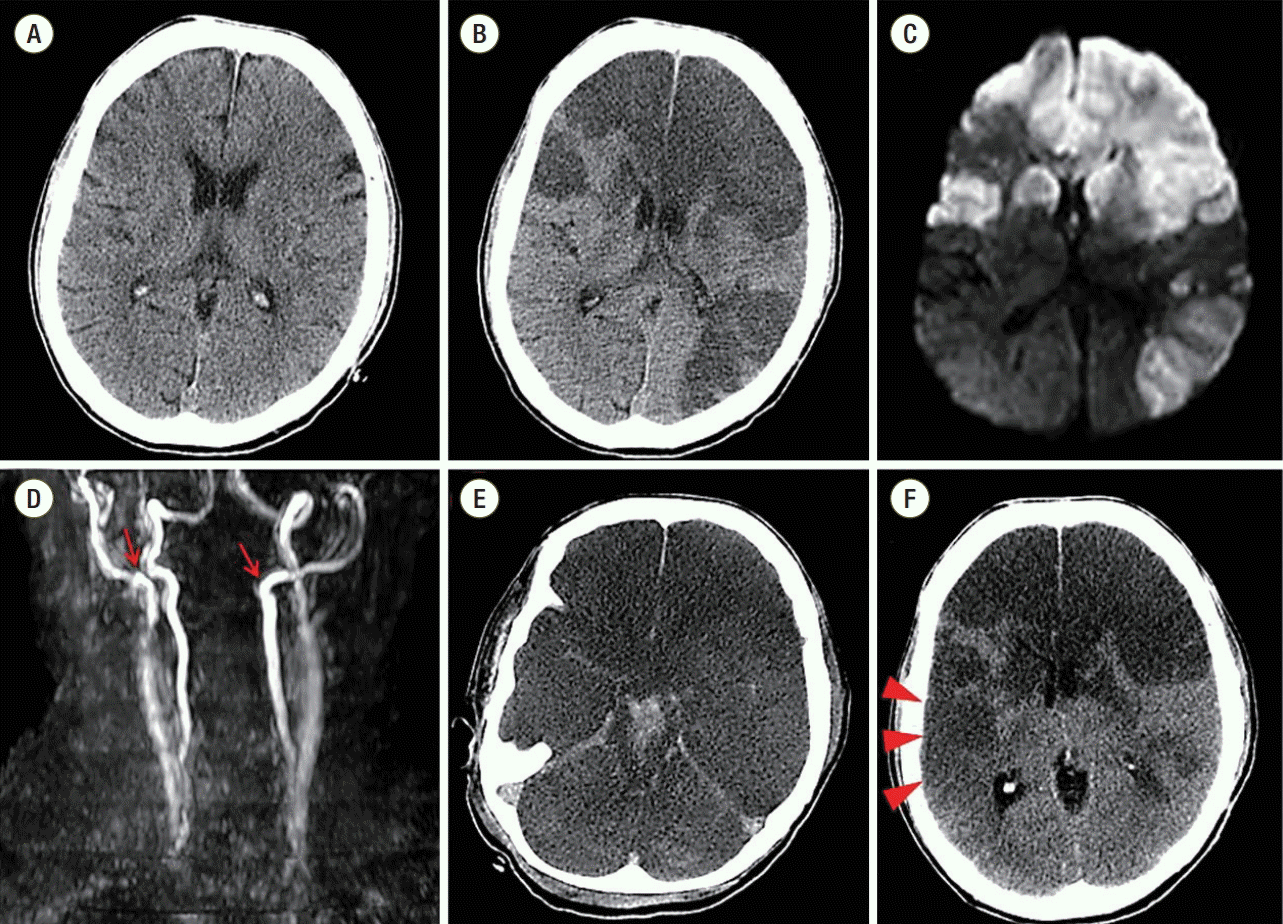
Fig. 3.Electrocardiographic changes. During the stroke, electrocardiogram showed newly developed T inversions in multiple leads (B) compared to the exam at initial admission (A).
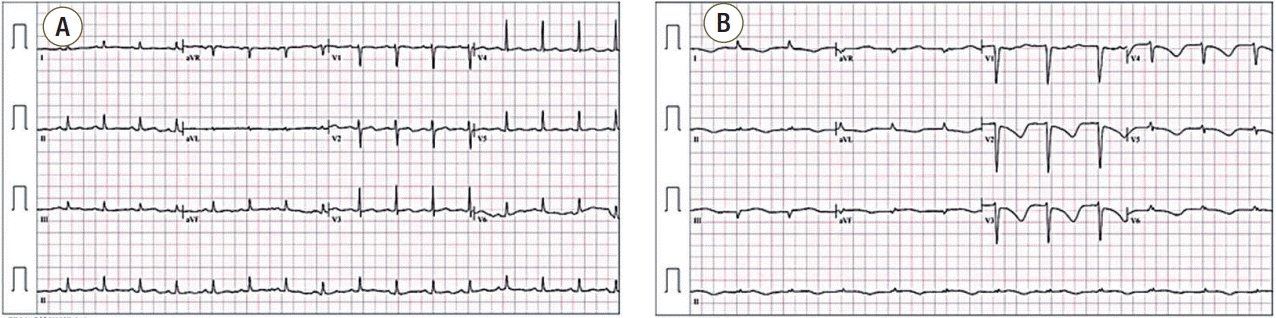
Fig. 4.Echocardiographic findings. Transthoracic echocardiography showed a large mural thrombus at the apex of the left ventricle (red arrowheads). Wall contraction at the base and mid-portion of the left ventricle was relatively preserved during systole compared to that of the left ventricular apex.
References
- 1. de Gregorio C. Cardioembolic outcomes in stress-related cardiomyopathy complicated by ventricular thrombus: a systematic review of 26 clinical studies. Int J Cardiol 2010;141:11-7.ArticlePubMed
- 2. Leick J, Szardien S, Liebetrau C, Willmer M, Fischer-Rasokat U, Kempfert J, et al. Mobile left ventricular thrombus in left ventricular dysfunction: case report and review of literature. Clin Res Cardiol 2013;102:479-84.ArticlePubMed
- 3. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol 2014;6:602-9.ArticlePubMedPMC
- 4. Madias JE. Why the current diagnostic criteria of Takotsubo syndrome are outmoded: a proposal for new criteria. Int J Cardiol 2014;174:468-70.ArticlePubMed
- 5. Pelliccia F, Greco C, Vitale C, Rosano G, Gaudio C, Kaski JC. Takotsubo syndrome (stress cardiomyopathy): an intriguing clinical condition in search of its identity. Am J Med 2014;127:699-704.ArticlePubMed
- 6. Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care 2014;29:500-11.ArticlePubMed
- 7. Singh K, Carson K, Shah R, Sawhney G, Singh B, Parsaik A, et al. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol 2014;113:1420-8.ArticlePubMed
- 8. Y-Hassan S, Settergren M, Henareh L. Sepsis-induced myocardial depression and takotsubo syndrome. Acute Card Care 2014;16:102-9.ArticlePubMed
- 9. Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006;152:75-84.ArticlePubMed
- 10. Leurent G, Larralde A, Boulmier D, Fougerou C, Langella B, Ollivier R, et al. Cardiac MRI studies of transient left ventricular apical ballooning syndrome (takotsubo cardiomyopathy): a systematic review. Int J Cardiol 2009;135:146-9.ArticlePubMed
Citations
Citations to this article as recorded by

- Spontaneous ventricular thrombosis in patients with inflammatory bowel disease
Stella Pak, Juan Linares, Yan Yatsynovich, David Cha, Dexter Nye, Diana Kaminski, Jillian Costello
Cardiology in the Young.2018; 28(3): 351. CrossRef - Major Trauma induced Left Ventricular Thrombus after Acute Myocardial Infarction
Dong Wook Lee, Ju Hee Ha, Jun Ho Kim, Ki Beom Park, Jae Joon Lee, Han Il Choi, Jin Hee Kim
Journal of Lipid and Atherosclerosis.2016; 5(2): 163. CrossRef
 , Doyeon Hwang, M.D.1, Chan-Soon Park, M.D.1, Jae-Sung Lim, M.D.2, Eungyu Kang, M.D.3, Joo-Hee Zo, M.D., Ph.D.1
, Doyeon Hwang, M.D.1, Chan-Soon Park, M.D.1, Jae-Sung Lim, M.D.2, Eungyu Kang, M.D.3, Joo-Hee Zo, M.D., Ph.D.1














 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite





