The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation
Article information
Abstract
Background
Anticoagulation during extracorporeal membrane oxygenation (ECMO) usually is required to prevent thrombosis. The aim of this study was to investigate the usefulness of nafamostat mesilate (NM) as a regional anticoagulant during veno-arterial ECMO (VA-ECMO) treatment.
Methods
We retrospectively reviewed the medical records of 16 patients receiving VA-ECMO and NM from January 2017 to June 2020 at Haeundae Paik Hospital. We compared clinical and laboratory data, including activated partial thromboplastin time (aPTT), which was measured simultaneously in patients and the ECMO site, to estimate the efficacy of regional anticoagulation.
Results
The median patient age was 68.5 years, and 56.3% of patients were men. Cardiovascular disease was the most common primary disease (75.0%) requiring ECMO treatment, followed by respiratory disease (12.5%). The median duration of ECMO treatment was 7.5 days. Among 16 patients, seven were switched to NM after first using heparin as an anticoagulation agent, and nine received only NM. When comparing aPTT values in the NM group between patients and the ECMO site, that in patients was significantly lower than that at the ECMO site (73.57 vs. 79.25 seconds; P=0.010); in contrast, no difference was observed in the heparin group.
Conclusions
NM showed efficacy as a regional anticoagulation method by sustaining a lower aPTT value compared to that measured at the ECMO site. NM should be considered as a safer regional anticoagulation method in VA-ECMO for patients at high risk of bleeding.
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a life-saving rescue therapy used to maintain cardiopulmonary function for critically ill patients. The use of ECMO has increased over the past few decades, and there is a growing demand for ECMO component technologies [1]. During ECMO treatment, exposure of blood to the large non-endothelial surface of the ECMO circuit, blood pump, and oxygenator system based on the type of ECMO causes contact activation and an associated risk of thrombus formation within the circuits or human circulation [2]. Therefore, effective systemic anticoagulation is usually required. In addition to the use of anticoagulation, circuit-related clotting factors, platelet consumption, and coagulopathy-associated critical illness can also increase the bleeding risk [3]. Thrombosis and bleeding are the most frequent and serious complications of ECMO and are associated with a high risk of mortality [4].
Among anticoagulants, unfractionated heparin (UFH) is one of the most widely used and well-studied anticoagulants during ECMO support given its short half-life and reversibility by protamine [5]. However, systemic anticoagulation achieved by heparin use exposes patients to a risk of bleeding due to UFH overdose, thrombocytopenia, platelet dysfunction, coagulopathy, and fibrinolysis [6]. Especially, heparin-induced thrombocytopenia is associated with high morbidity and mortality rates [7]. Titrating the intensity and balance of systemic anticoagulation between thrombosis and bleeding is very important and remains a major challenge.
Nafamostat mesilate (NM) is a synthetic serine protease inhibitor that has been used primarily for anticoagulation in patients with continuous renal replacement therapy (CRRT) and ECMO in Japan and Korea [8]. NM is an effective anticoagulant and was anticipated to reduce the adverse event of bleeding during blood purification in critically ill patients due to its short half-life [9-11]. However, the actual efficacy and safety of NM as a regional anticoagulant in patients receiving ECMO have not been well demonstrated. Therefore, the aim of this study was to evaluate the efficacy and safety of NM as a regional anticoagulant in Korean patients undergoing veno-arterial ECMO (VA-ECMO).
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Haeundae Paik Hospital (IRB No. 2020-06-016), and the requirement for written informed consent was waived due to the retrospective nature of this study.
Study Participants
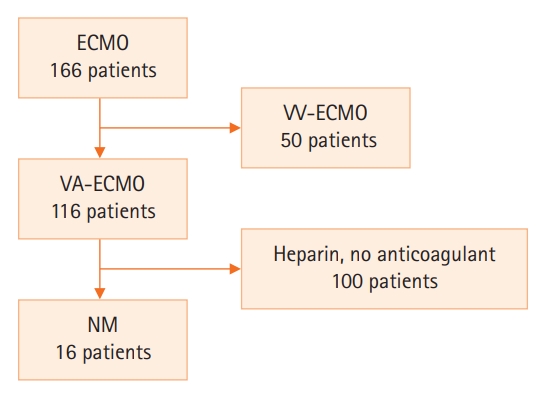
Among 116 patients who were treated with VA-ECMO (166 in total, with 50 receiving veno-venous ECMO [VV-ECMO]), 16 with available clinical and laboratory data and who were treated with NM as an anticoagulant from May 2017 to June 2020 at Haeundae Paik Hospital, Korea, were analyzed retrospectively. Patients who underwent activated partial thromboplastin time (aPTT) testing at 6-hour intervals during ECMO were included in the analysis (Figure 1).
Clinical Information
Baseline characteristics and laboratory data were obtained from medical records. The primary disease requiring ECMO treatment and the presence of adverse events, including bleeding before and during ECMO application, were recorded. The Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated to classify disease severity for adult patients admitted to the intensive care unit (ICU), and the Simplified Acute Physiology Score (SAPS) III was measured to predict ICU mortality.
ECMO Apparatus
The Permanent Life Support (PLS) system by Maquet (Rastatt, Germany), consisting of a PLS-i oxygenator with a Bioline coating and a Rotaflow centrifugal pump (RF-32), was the ECMO system used. The PLS circuit was primed with 1 L of normal saline or plasma solution, and the total circuit volume was 500 to 600 mL.
Anticoagulation and Assessment of Regional Anticoagulation Effects
NM (SK Chemicals Life Science, Seongnam, Korea; licensed by Toril Pharma, Tokyo, Japan) was infused continuously through an exclusive stopcock installed in the drainage route before the ECMO pump. NM was started at a rate of 0.2 to 0.5 mg/kg/hr without bolus injection. The maintenance dose of NM was regulated to achieve an aPTT range of 60 to 90 seconds, as measured by Sysmex CA-7000 (Siemens, Munich, Germany). The patient and ECMO site samples were obtained at the central venous catheter and the circuit of ECMO after the oxygenator and pump, respectively. We compared differences in aPTT at 6-hour intervals to identify the usefulness of NM as a regional anticoagulation agent. Enrolled patients were subdivided into an NM group and a heparin group that was treated with heparin before switching to NM to compare the differences in regional anticoagulation.
Statistical Analysis
The data are presented as frequencies with percentages for categorical variables and as median and interquartile range values for continuous variables. A paired t-test or Wilcoxon’s signed-rank test was performed for comparison between two time points. To determine the normality of data distribution, the Shapiro-Wilk test was used. Line graphs were presented for data visualization. All statistical analyses were carried out using the IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA), and P-values less than .05 were considered to be statistically significant.
RESULTS
Baseline Clinical Characteristics
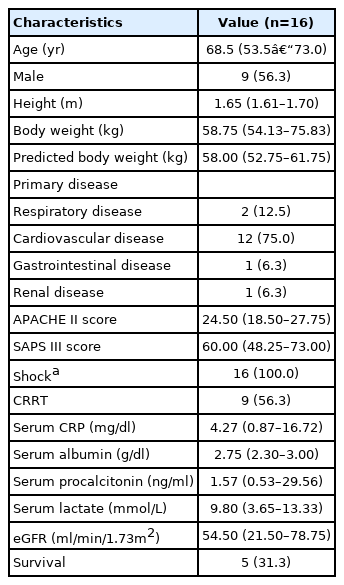
A total of 16 patients was included in this study (Table 1). The median age was 68.5 years, and 56.3% of patients were men. Cardiovascular disease was the most common primary disease (75.0%) requiring ECMO treatment, followed by respiratory disease (12.5%). All patients existed in a shock state, defined as the use of an inotropic agent or vasopressor to maintain adequate tissue perfusion (mean arterial pressure >65 mm Hg), and nine patients (56.3%) were treated with CRRT. The median APACHE II and SAPS III scores were 24.5 and 60.0 points, respectively. Before ECMO treatment, one patient suffered a bleeding situation at a postoperative site, but there were no thrombotic adverse events recorded during the study.
Details of VA-ECMO and Anticoagulation
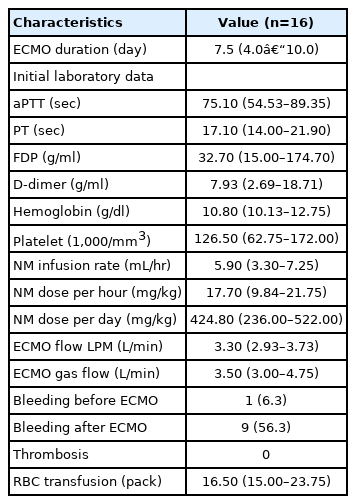
Among 16 patients, seven initially were given heparin as an anticoagulant agent and switched to NM due to bleeding during ECMO. Nine patients originally received NM as an anticoagulant agent because of a high risk of bleeding before ECMO treatment and existing postoperative bleeding. All patients were cannulated with arterial and venous cannulae in both femoral vessels according to body size. The median duration of ECMO treatment was 7.5 days (range, 4.0–10.0 days). The median flows of ECMO and gas were 3.3 L/min (range, 2.9–3.7 L/min) and 3.5 L/min (range, 3.0–4.7 L/min), respectively. The median dose of NM was 17.7 mg/hr (range, 9.8–21.7 mg/hr) and 424.8 mg/day (range, 236.0–522.0 mg/day). The details of VA-ECMO apparatus and anticoagulation are summarized in Table 2.
Analysis of aPTT and Complications between Patient and ECMO Site
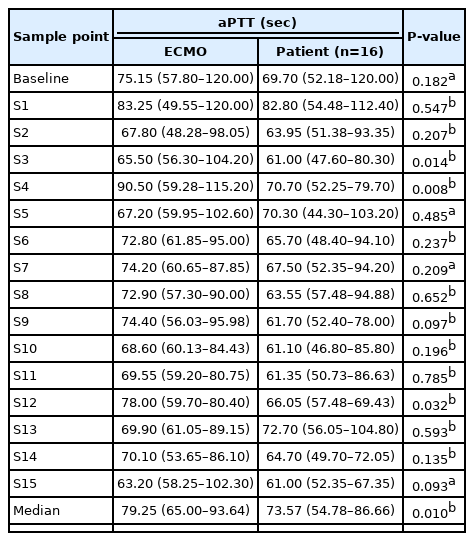
The aPTT values at 16 consecutive sample points during a six-hour interval between patients and the ECMO site were compared. The aPTT analysis of the NM group included data from all 16 patients, and the analysis of the heparin group included data from seven patients who received heparin before being switched to NM. In these 16 patients who received NM, the pooled aPTT value of patients was significantly lower than that of the ECMO site (median aPTT, 73.57 vs. 79.25 seconds; P=0.010) (Table 3, Figure 2A). However, there was no difference between patients and ECMO among those given heparin before NM (median aPTT, 72.84 vs. 72.95 seconds; P=0.768) (Table 4, Figure 2B). In patients given NM, the target aPTT was achieved at most sample points. During a subgroup analysis in those who received both heparin and NM, the pooled aPTT value of patients was significantly lower than that of the ECMO site during NM use (median aPTT, 68.42 vs. 73.13 seconds, P=0.031) (Table 4, Figure 2C).
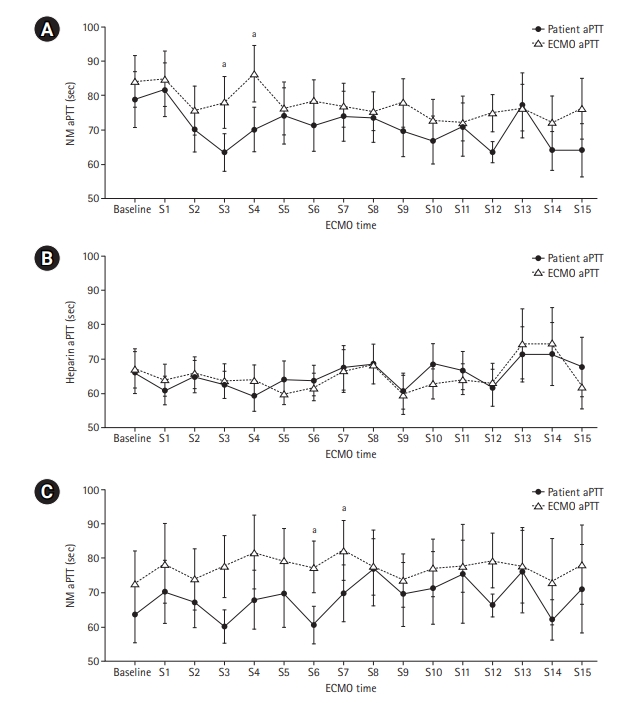
Comparison of activated partial thromboplastin time (aPTT) values between patients and extracorporeal membrane oxygenation (ECMO) site. (A) The pooled aPTT value in patients was significantly lower than that of the ECMO site in those receiving nafamostat mesilate (NM). (B) The pooled aPTT value was not different between patients and the ECMO site in those receiving heparin before NM. (C) The pooled aPTT value in patients was significantly lower than that of the ECMO site in those receiving NM after heparin. S: sample point. aIndicates statistically significant differences between patients and the ECMO site at each sample point.
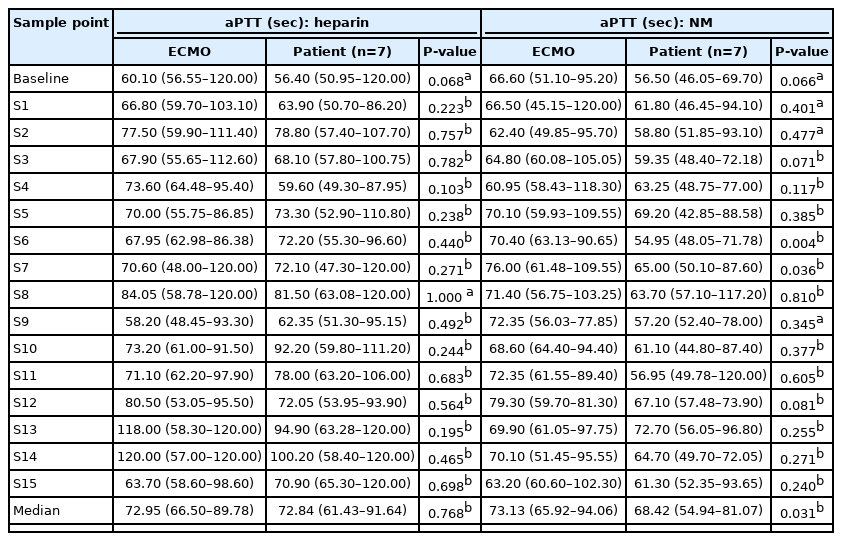
Comparison of pooled level of aPTT during VA-ECMO in patients receiving both heparin and NM as anticoagulant drug
Among seven patients originally treated with heparin, six suffered adverse events of bleeding during ECMO treatment (including four with cannulation site bleeding, one with gingival bleeding, and one with hematochezia); after changing to NM, this bleeding improved in all patients. In nine patients originally receiving NM, three suffered bleeding events (including two with postoperation site bleeding and one with hemothorax after bedside needle thoracentesis); however, none of these cases were severe, showed substantial hemodynamic compromise, or required transfusion. There was no difference in the quantity of red blood cell transfusion between the two groups. Also, there were no adverse events, such as drug or hypersensitivity reactions, associated with NM.
DISCUSSION
In our study, NM showed efficacy as a regional anticoagulant during ECMO treatment. aPTT values in patients were significantly lower than that of the ECMO site; further, no adverse event of thrombosis occurred, and clinically significant bleeding was reduced in patients with NM compared to those treated with heparin. Also, at most time points, the target aPTT value was achieved in the NM group without significant adverse events.
During ECMO treatment, systemic anticoagulation is usually recommended to prevent thrombosis resulting from the intrinsic nature of ECMO, including exposure of blood to a non-endothelial biosurface and a complement-mediated inflammatory response [12]. Especially, the risks of thrombosis and thromboembolic events can increase in patients with VA-ECMO due to the turbulent flow of circulation and left ventricular stasis [6,13,14]. However, systemic anticoagulation might present a risk for clinically relevant bleeding and anticoagulant-derived side effects [15,16]. Despite the growing use of ECMO and continued technologic advances, hemorrhagic and thrombotic complications account for the majority of mortality and morbidity events in patients undergoing ECMO [17]. Maintaining optimal hemostasis and a good balance between thrombosis and bleeding is key for managing patients safely during ECMO and is associated with better clinical outcomes. Therefore, this study was conducted because we hypothesized that regional anticoagulation is a more important and safer method than systemic anticoagulation in patients undergoing VA-ECMO.
NM is a serine protease inhibitor that has anticoagulant, antifibrinolytic, and antiplatelet actions [18]. Also, in Japan and Korea, NM has been used as an alternative anticoagulant in patients at high risk of bleeding during hemodialysis because of its very short half-life [10,19]. NM has a very short biological half-life, approximately five to eight minutes, compared to that of UFH, and is inactivated by hydrolysis catalyzed by carboxylesterase in the blood during extracorporeal blood purification [20,21]. Some studies support the results that NM provides sufficient filter survival and reduces adverse bleeding events during CRRT in patients at high risk of bleeding [22,23].
Because hemodialysis and ECMO have similar mechanisms, there have been attempts to use NM during ECMO. In our study, NM led to a significant difference in aPTT value between patients and the ECMO site, suggesting the usefulness of NM as a regional anticoagulant and its similar anticoagulation effect compared to that of heparin. In a previous study, Han et al. reported that, among 90 patients undergoing ECMO (including 22 receiving heparin and 68 receiving NM), the NM group experienced fewer bleeding complications compared to the heparin group without an increased incidence of thrombosis (bleeding, 38.2% vs. 72.7%; P=0.005) [24]. In this study, heparin use was a significant risk factor for bleeding (hazard ratio, 4.372; 95% confidence interval, 1.449–13.190; P=0.009). In 13 patients with ECMO (including six receiving VA-ECMO and seven receiving VV-ECMO), Park et al. [25] reported that the activated clotting time (ACT) and aPTT at the patient site were significantly lower than those at the ECMO site during VA-ECMO; the same finding was not observed in those patients on VV-ECMO. These authors suggested that the reason for the lack of differences observed in ACT and aPTT values in the VV-ECMO population might be because NM enters directly into the hepatic circulation and is metabolized quickly by the liver before it is subjected to dilution by the systemic blood flow during VV-ECMO. Also, their study included seven patients who used both heparin and NM and showed equivalent efficacy of regional anticoagulation of NM. This demonstrates the usefulness of NM as a regional anticoagulant in such patients.
In addition to the effect of regional anticoagulation, NM has been reported to have the benefits of a protective effect against disseminated intravascular coagulation in an endotoxin-administered rat model as well as an anti-inflammatory effect [26,27]. Recently, in 268 sepsis patients who received NM and conventional treatment, Kamijo et al. [28] reported that the use of NM significantly reduced ICU and hospital mortality rates in sepsis patients who underwent blood purification compared to those treated with other anticoagulants. Since most patients who require ECMO treatment present with severe conditions, such as sepsis, coagulopathy, and disseminated intravascular coagulation, NM might be a more useful treatment.
This study has some limitations. First, this was a retrospective study involving only a small number of patients from a single center. However, the total number of samples was sufficient to produce statistical significance, which was noted in the comparison of aPTT values between patients and the ECMO site. Second, only an aPTT assay was used to determine the efficacy of regional anticoagulation in this study. The efficacy of anticoagulation can be monitored by aPTT, ACT, and anti-Xa assay; however, ACT does not represent only the effect of anticoagulant and might be affected by other variable conditions, and the means to perform an anti-Xa assay were not available at our institution. In addition, the aPTT test is recommended by the Extracorporeal Life Support Organization and has been most widely used. Therefore, in the future, studies using other test methods, such as an anti-Xa assay and viscoelastic tests, are needed to estimate the efficacy of NM as a regional anticoagulant. Third, this study included anticoagulation data only from patients undergoing VA-ECMO. Thus, more studies considering regional anticoagulation in patients undergoing VA-ECMO or VV-ECMO are needed in patients at high risk of bleeding requiring ECMO treatment.
In conclusion, NM showed usefulness as a regional anticoagulation method in patients on VA-ECMO. The aPTT values in patients were significantly lower than that of the ECMO site, with clinically fewer adverse bleeding events. In patients on VA-ECMO with bleeding who are receiving heparin or who are at high risk of bleeding, NM should be considered as a regional anticoagulant.
KEY MESSAGES
▪ Nafamostat mesilate showed efficacy as a regional anticoagulation method by sustaining a lower activated partial thromboplastin time (aPTT) value compared to that measured at the extracorporeal membrane oxygenation (ECMO) site during veno-arterial ECMO (VA-ECMO).
▪ Nafamostat mesilate should be considered as a regional anticoagulation alternative for patients at high risk of bleeding during VA-ECMO.
Notes
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: JHL, HJJ. Data curation: JHP, JHJ, SHK, WH, KHK. Formal analysis: SYH, SHK, DHL, KHK. Methodology: SYH, SHK, DHL, KHK. Visualization: JHL, SYH. Writing–original draft: JHL, HSC, JHP. Writing–review & editing: all authors.
Acknowledgements
The authors would like to thank Hae Woong Park, Che Eun Im, Yoon-Sung Choi, See Won Choe, and Seo Hyeon Jang (perfusionists in the Department of Cardiac Surgery at Haeundae Paik Hospital) for their contributions and efforts in the care of patients on extracorporeal membrane oxygenation.