Toxic Shock Syndrome following Tattooing
Article information
Abstract
Toxic shock syndrome (TSS) is a rare but life-threatening illness that is mainly caused by toxigenic strains of Staphylococcus aureus. Although TSS is classically known to be associated with tampon use, the number of TSS cases with non-menstrual causes such as skin and soft tissue infection has been increasing. Tattooing can result in several complications such as localized and systemic infections, inflammatory skin eruptions and neoplasms. We recently experienced a 26-year-old man diagnosed with typical TSS following tattooing. He complained of fever, chills and erythematous rash at tattoo site. Subsequently, the patient developed sign of shock. The skin cultures on the tattoo site were positive for methicillin-sensitive Staphylococcus aureus. The patient was successfully treated with vasopressor infusion and intravenous antibiotics and was discharged without complications. On discharge from the hospital 7 days later, desquamations on the tattoo site, fingers and toes were observed.
Toxic shock syndrome (TSS) is a rare but life-threatening illness that is mainly caused by toxigenic strains of Staphylococcus aureus (SA). The clinical feature of it is characterized by fever, skin rash, hypotension, desquamation and multi-organ failure.[1] Although classically known to be associated with women using tampon, the incidence of these menstrual TSS has substantially decreased with increasing market reform and education for proper use of it.[2] On the contrary, the number of non-menstrual TSS related with skin and soft tissue as the primary infection sites has relatively increased.[3-4]
Tattoo, an indelible mark of body made by deposition of ink into the dermis layer of the skin, has developed several complications such as localized and systemic infections, inflammatory skin eruptions and neoplasms.[5] Its complication rate was approximately 2-3% and most are related to infections. The most severe complications which can lead to death are systemic infections such as septicemia, TSS and endocarditis, but these are very rare.[6]
Herein, we report a recently encountered case of TSS caused by SA after tattooing in a patient who had no underlying disease.
Case Report
A 26-year-old man visited the emergency department with complaints of a fever, chill, myalgia, headache and diarrhea over 3 days. History revealed that these symptoms were developed after receiving the tattoo on the back 3 days prior to presentation. He presented with no significant past medical history and intravenous drug use. On presentation, he was alert but lethargic and sweating. Initial vital signs were blood pressure 151/57 mmHg, heart rate 140 beats/min, respiratory rate 20/min, body temperature 38°C and pulse oximetry 99% on room air. Physical examination was remarkable for erythematous rash and multiple greenish papules at the patient’s tattoo sites (Fig 1A, 1B), as well as non-itchy erythematous rash on the anterior chest wall (Fig. 1C).
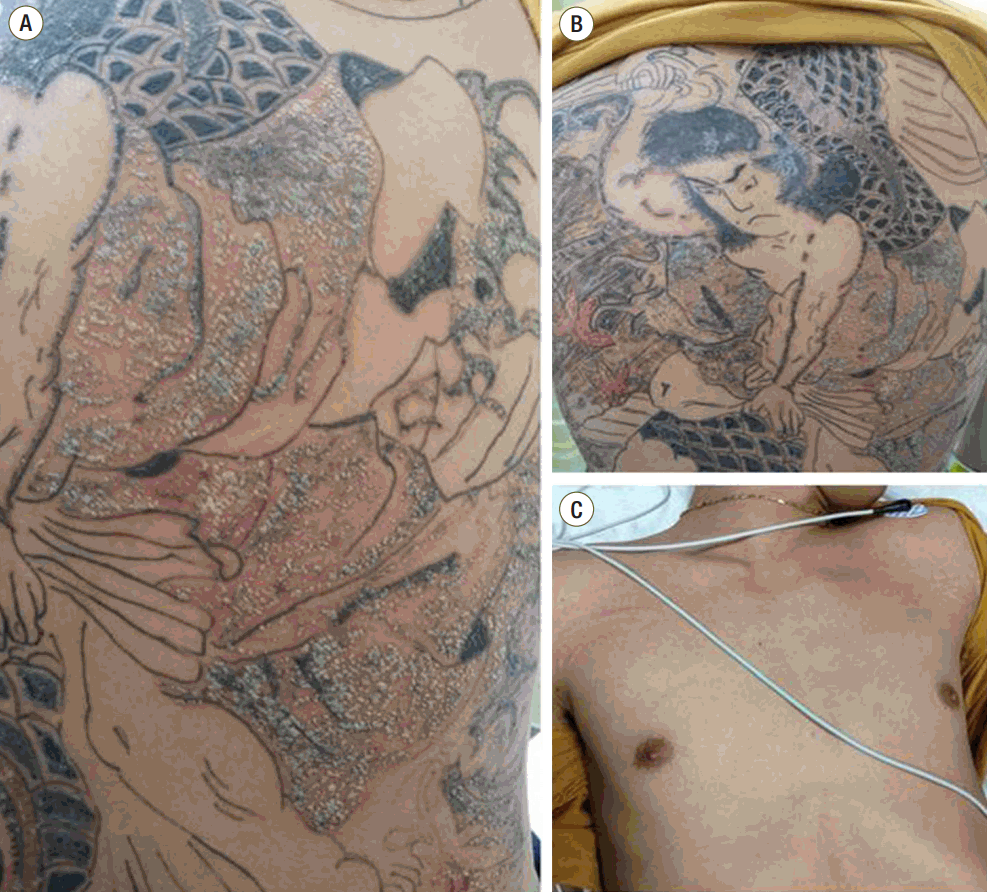
Skin lesions around the tattoo sites three days after tattooing (A and B), and erythematous rash on the anterior chest wall (C).
While blood samples were taken, he began to feel drowsy and subsequently became confused. At this time, blood pressure and heart rate were 73/36 mmHg and 123 beats/min, respectively. An electrocardiogram showed sinus tachycardia. The patient was immediately transferred to the resuscitation room for management of shock. Rapid fluid resuscitation was instituted and the central venous and arterial catheters were inserted into the right internal jugular vein and radial artery, respectively. He was admitted to the emergency intensive care unit. Because the patient had hypotension that was refractory to fluid resuscitation (2 L over 90 min), subsequent infusion of norepinephrine was required to maintain the mean arterial blood pressure above 70 mmHg.
Abnormal laboratory investigations on admission showed a white blood cell count of 25,940/μL (77% of neutrophils), blood urea nitrogen 31 mg/dL, creatinine of 1.77 mg/dL, total bilirubin of 1.3 mg/dL, aspartate aminotransferase of 171 IU/L, alanine aminotransferase of 170 IU/L and C-reactive protein of 27.34 mg/dL. A prothrombin time, international normalized ratio, activated partial thromboplastin time and fibrinogen levels were elevated to 14.8 s, 1.38, 39.7 s and 534 mg/dL, respectively, and urine examination revealed 1+ albuminuria and pyuria (20-29 leukocytes under high power field). In addition, serum HBs Ag, anti-HCV, anti-HIV and rapid plasma reagin were all negative and the parameters of arterial blood gas analysis were within the normal range. His platelet count on admission was normal, but decreased to 115 × 103/μL 6 hours later. A urine culture and two blood cultures were drawn upon admission. A skin culture, a swab from exudative cutaneous lesions on the tattoo site, was also performed.
Empirically, intravenous infusion of vancomycin 1 g q 12 hr, ceftriaxone 2 g per 24 hr and clindamycin 600 mg q 8 hr were started for presumed TSS and for the procedure related gram negative infections. After 8 hours, the patient was hemodynamically stable and had recovered normal mentation. One day later, he continued to require norepinephrine to maintain hemodynamic stability, but with tapering dose of it, and gram-positive cocci were observed in a skin culture. On day 3, those from a skin culture were confirmed as methicillin-sensitive SA (MSSA), but blood cultures obtained on admission were negative. And then, the intravenous antibiotic regimen was narrowed to cefazolin (2 g q 8 hr) and clindamycin (600 mg q 8 hr). A fluctuant fever, with a peak temperature of 40.1℃, had persisted for 2 days after admission and subsided on the 3rd hospital day. His condition improved and his laboratory test gradually normalized. Intensive supportive care including norepinephrine treatment was withdrawn on the 3rd hospital day and he was transferred to a general ward.
Desquamations of tattoo sites and fingers were found on day 4 and 6, respectively. The patient was treated with intravenous antibiotics for a total of 7 days and was discharged on oral cephradine for a further 7 days. At 3 days after discharge, the patient was clinically asymptomatic, except for desquamations on the fingers and toes (Fig. 2). Oral cephradine (500 mg q 6 hr) were continued for one week after discharge.
Discussion
Tattoo has become increasingly popular and aesthetic procedures easily available in recent years. Recent surveys have shown an increase in tattoo rates, but these are different for each nation, affiliation and age: 24% within the US population aged 18 to 50 years old, 10% within the Australian population aged 14 years and over, 8.5% and 15% within the German population aged 14 to 93 and 14 to 44 years old, respectively, 16 to 23% and 9.6% of US and Italian college students, respectively, 27 to 57% of US military recruits and 4.5% and 6% of US and Italian adolescents, respectively.[5,7-10] With an increasing incidence of tattooing, especially in adolescents and young adults, the rate of tattoo-associated complication increases. In addition, it was known that the number and size of tattoo and the age of first tattoo placement were associated with the development of its complications.[10] Numerous tattoo-associated complications have been reported as follows: (1) inflammatory complications including lichenoid reaction, eczematoid, granuloma, vasculitis, urticarial, anaphylaxis, lupus and dermatofibroma; (2) infectious complications including impetigo, erysipelas, methicillin-resistant SA (MRSA), non-tuberculous mycobacteria, cutaneous tuberculosis, leprosy, syphilis, molluscum contagiosum, hepatitis B virus, hepatitis C virus, human immunodeficiency virus and bacterial endocarditis; (3) neoplastic complications including melanoma, keratoacanthoma, squamous cell carcinoma, basal cell carcinoma and leiomyosarcoma.[5] Among them, most complications are related to local and systemic infections. Three potential sources of these infectious complications after tattooing were reported: (1) the contaminated tattoo ink itself, especially by bacterial pathogens; (2) the penetration of residential bacteria following an insufficient sterilization of the skin during tattooing process; (3) superinfection of the injured tissue with tattoo during the healing stage after tattooing.[6] However, unlikely local infections such as impetigo, erysipelas and ecthyma, the incidence of severe systemic complications which may lead to death is relatively very low. When reviewing the medical literature on severe bacterial infections after tattooing, there are only 9 documented cases during 1993 to 2014: a case of TSS, 4 cases of necrotizing infections, a case of epidural abscess, 2 cases of bacterial endocarditis and a case of polymicrobial septicemia.[11-18]
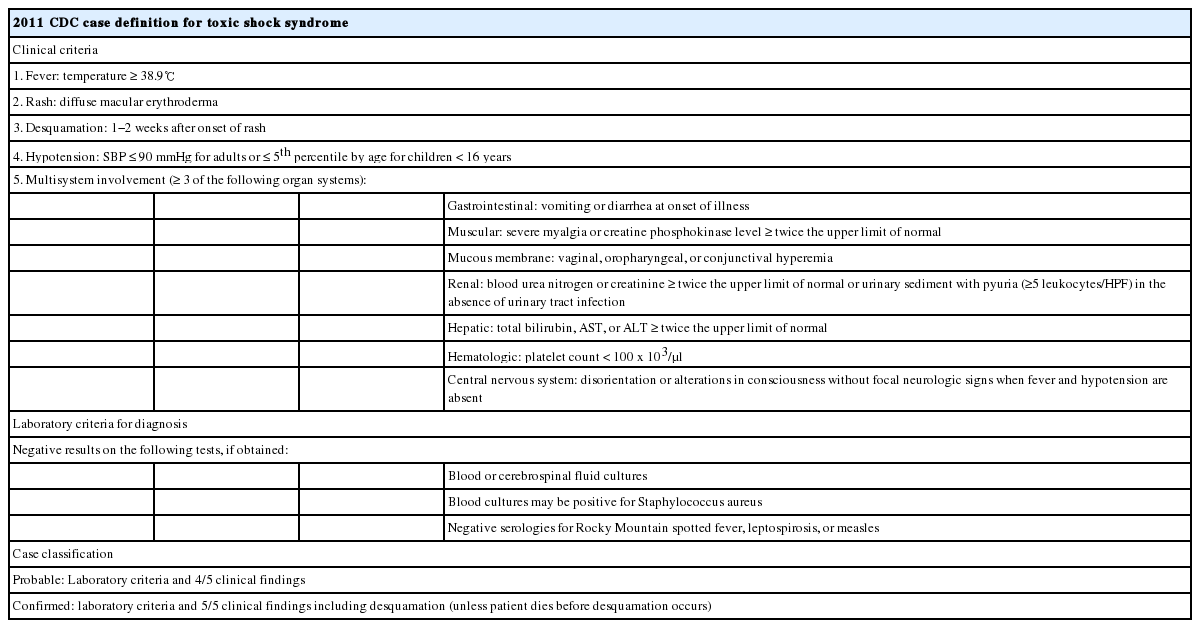
TSS which was first described in 1978 is an acute, toxin-mediated illness leading to multi-organ failure with serious mortality. Although superantigens produced by strains of SA including toxic shock syndrome toxin-1 (TSST-1) and enterotoxins A-E are known as causes of TSS, most cases of it were caused by TSST-1.[1-3] TSST-1 which suppresses neutrophil chemotaxis leads to induce T-suppressor function and simultaneously stimulates the release of several cytokines that is responsible for the signs and symptoms of the syndrome.[5] The 2011 Center for Disease Control and Prevention (CDC) diagnostic criteria for TSS are presented in Table 1. Among clinical manifestation of TSS, the typical signs and symptoms are a high fever over 38.9°C, headache, vomiting, diarrhea, myalgias, and a diffuse macular rash characterized as sunburn. Our case undoubtedly meets the ‘confirmed’ criteria for TSS within the CDC guidelines: fever, rash, desquamation, hypotension, diarrhea, myalgia, increase of creatinine and liver enzyme, thrombocytopenia, and alteration in consciousness without focal neurologic sign in the setting of negative blood culture.
TSS is subdivided into menstrual TSS and non-menstrual TSS. Menstrual TSS is almost associated with tampon use and strains of SA producing TSST-1.[1] In menstrual-aged women, the peak incidence of menstrual TSS was reported to be 6.2-12.3 cases/100,000/year in the early1980s, but, since that time, the number of cases declined to 1 cases/100,000/year in the late 1980s. Recent study during 2000-2003 showed that the annual incidence of total TSS (both menstrual TSS and non-menstrual TSS), menstrual TSS, and non-menstrual TSS was 0.52, 0.69 and 0.32 per 100,000 populations, respectively.[2,3,19] In addition, there was no significant increase in incidence rates of these during 2000-2006. Contrary to menstrual TSS, it is known that non-menstrual TSS is more often associated with several infectious conditions such as pneumonia, abscess, sinusitis and cellulitis and surgical procedures. And, it is mainly caused by strains of SA producing either TSST-1 or enterotoxin B or C.[2] In the late 1980s, the yearly incidence of non-menstrual TSS was 0.3/100,000 populations and it has not changed significantly over the last 20 years.[3] However, The proportion of non-menstrual TSS cases among total TSS cases gradually appeared to be on the increase. Although the proportion of non-menstrual TSS accounted for 29% during 1981-1986, it has been quoted at up to 62% of total TSS cases after 1986; Hajjeh et al.[19] reported 41% of non-menstrual TSS during 1987-1996, DeVries et al.[3] reported 46% of it during 2000-2006, and Descloux et al.[20] reported 62% of it during 2003-2006, respectively. The clinical features of menstrual TSS and non-menstrual TSS are mostly identical. However, a previous study reported that patients with non-menstrual TSS may have the delayed onset of symptoms and a more frequent involvement of renal and central nervous system, while a musculoskeletal involvement may be less frequent.[4] The onset of initial symptoms occurred within 24 hours following tattooing in our non-menstrual TSS case and it was differ from literature data.
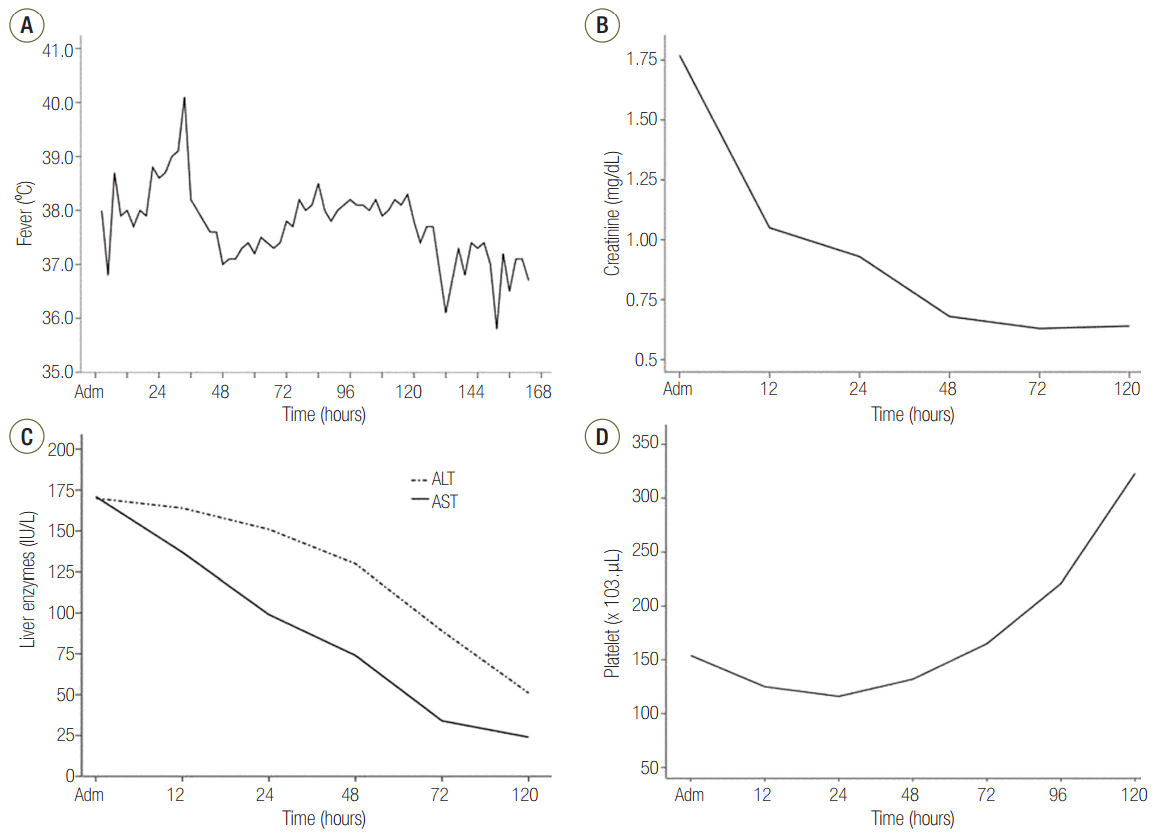
Although the onset of initial symptoms following tattooing was earlier, the patient in our case followed a typical clinical course of TSS. The first symptoms before admission mimicked a gastroenteritis and influenza, fever resolved by day 5-6 after initial symptoms (Fig. 3A), skin change with erythematous rash was observed on day 3, and desquamation was shown on day 7-9. Abnormal laboratory findings returned to baseline or normal within 7-8 days after the onset of symptoms (Fig. 3B for creatinine levels, Fig. 3C for liver enzyme levels and Fig. 3C for platelet counts). In addition, a skin culture of tattooing site grew MSSA, but blood cultures were negative. It was reported that the proportion of positive blood cultures is below 5% in staphylococcal TSS cases, while cultures from infected sites are usually positive.[19] Although strains of MSSA are still responsible for the wide range of staphylococcal infections, from minor skin infections to severe systemic illness, community-associated MRSA strains have increased in prevalence during the last 20 years. It is notable that MRSA have emerged as a causative organism of non-menstrual TSS associated with skin and soft tissue infection.[3] For this reason, administration of broad-spectrum empirical antibiotics including vancomycin was promptly commenced. MSSA was isolated from the skin culture of tattooing site in our case, but subsequently toxin gene analysis was not performed.
In conclusion, tattoo-associated complications have increased along with the growing popularity and social acceptability of it. We described a very rare case of staphylococcal TSS following tattooing and reviewed tattoo and TSS which is one of its systemic complication in the medical literature. Despite the rarity of TSS following tattooing, ED physicians must not only be able to recognize tattoo-associated TSS, but promptly provide better treatment for patients with it.
Notes
No potential conflict of interest relevant to this article was reported.