Diet-related complications according to the timing of enteral nutrition support in patients who recovered from out-of-hospital cardiac arrest: a propensity score matched analysis
Article information
Abstract
Background
A proper nutritional plan for resuscitated patients is important in intensive care; however, specific nutritional guidelines have not yet been established. This study aimed to determine the incidence of diet-related complications that were affected by the timing of enteral nutrition in resuscitated patients after cardiac arrest.
Methods
This retrospective and 1:1 propensity score matching study involved patients who recovered after nontraumatic, out-of-hospital cardiac arrest at a tertiary hospital. Patients were divided into an early nutrition support (ENS) group and a delayed nutrition support (DNS) group according to the nutritional support time within 48 hours after admission. The incidence of major clinical complications was compared between the groups.
Results
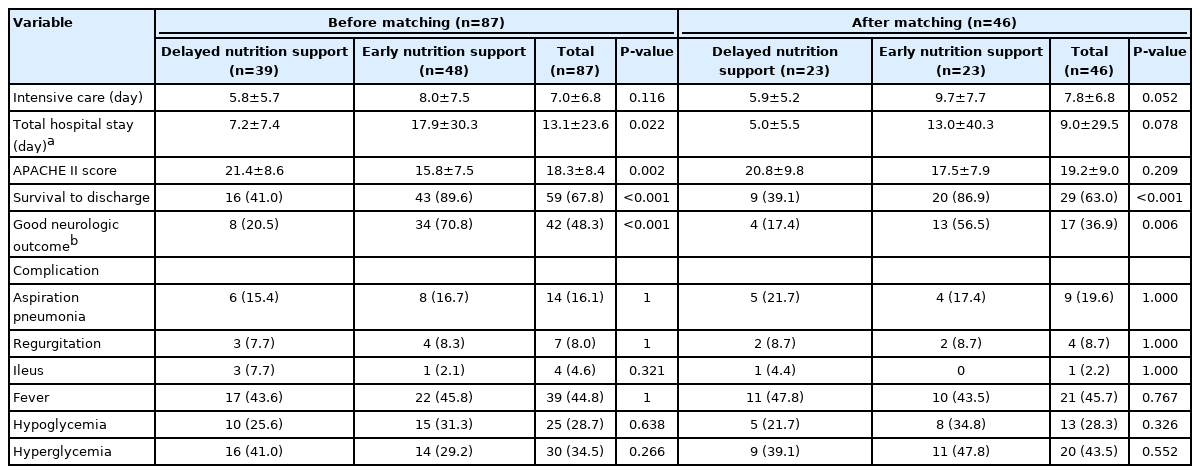
A total of 46 patients (ENS: 23, DNS: 23) were enrolled in the study. There were no differences in body mass index, comorbidity, and time of cardiopulmonary resuscitation between the two groups. There were 9 patients (ENS: 4, DNS: 5) with aspiration pneumonia; 4 patients (ENS: 2, DNS: 2) with regurgitation; 1 patient (ENS: 0, DNS: 1) with ileus; 21 patients (ENS: 10, DNS: 11) with fever; 13 patients (ENS: 8, DNS: 5) with hypoglycemia; and 20 patients (ENS: 11, DNS: 9) with hyperglycemia. The relative risk of each complication during post-resuscitation care was no different between groups.
Conclusions
There was a similar incidence of diet-related complications during post cardiac arrest care according to the timing of enteral nutrition.
INTRODUCTION
Patients who recover spontaneous circulation after cardiac arrest are among the most complex patients requiring intensive care [1]. Considering the various clinical situations that occur during the treatment of resuscitated patients after cardiac arrest, including shock conditions, clinicians may use crystalloid fluid, vasopressors, antibiotics for infection control, targeted temperature management, renal replacement therapy, or extracorporeal membrane oxygenation [2-5]. Many patients take time to recover from shock, and if the target temperature management is provided or the patient's movement is controlled by administering a sedative or muscle relaxant, it can also affect bowel movements [6-8].
Patients admitted to the intensive care unit (ICU) are often in critical condition or in severely stressful situations, which can increase their metabolic rates and energy requirements [9]. Supporting nutrition can prevent metabolic degradation and reduction of body mass in critically ill patients, weaken the metabolic response and control the immune response favorably through proper nutrition, reduce the patient's hospital stay, and decrease morbidity [10].
For various types of critically ill patients, specific guidelines from the Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN) are recommended [11]. Nevertheless, for post-resuscitation patients after cardiac arrest, specific guidelines for nutritional supplementation have not yet been properly established, other than the guidelines for maintaining blood sugar levels of 144–180 mg/dl during targeted temperature management [12]. Enteral nutrition is often not considered a priority in clinical settings due to symptomatic issues such as vulnerable cardiovascular conditions or the need for intensive mechanical ventilation [13]. Moreover, it can be clinically burdensome to apply enteral nutrition prematurely for post-resuscitated patients. In addition, since the benefits of nutritional support are not yet confirmed, clinicians are concerned about when to administer nutritional supplements during the treatment process and what type of nutritional supplements to choose [14]. Thus, it is essential to determine a proper time for supporting enteral nutrition and to identify clinical conditions including possible complications noted in each post-resuscitated patient.
This study was performed to evaluate the incidence of diet-related complications according to the timing of enteral nutrition in patients who experienced return of spontaneous circulation after out-of-hospital cardiac arrest (OHCA).
MATERIALS AND METHODS
Ethics Statement
The study design was approved by the Institutional Review Board of Wonju Severance Christian Hospital, Yonsei University (No. CR320098). The requirement for obtaining informed consent was waived by the Institutional Review Board because of the retrospective nature of the study.
Study Design
This retrospective observational study was conducted in patients with return of spontaneous circulation after non-traumatic OHCA and who were admitted to the ICU of Wonju Severance Christian Hospital between October 2019 and June 2020. Inclusion criteria were age ≥19 years and admission to the ICU for post-cardiac arrest care. Patients who did not respond to verbal instructions after initial resuscitation continued to receive proper care in the ICU. Patients who had intestinal hemorrhage, were presumed to have ileus or intestinal obstruction on plain radiography, or had other reasons for an inability to tolerate enteral feeding were excluded.
Definition of Early Nutrition Support
The early nutrition support (ENS) group was defined as the group of patients who started enteral tube feeding within 48 hours after admission. Other cases of enteral nutrition support after 48 hours were defined as the delayed nutrition support (DNS) group.
Study Setting and Data Collection
When the patients with OHCA recovered spontaneous circulation after cardiopulmonary resuscitation in the emergency room, intensive care including targeted temperature management was continued in the ICU. Clinicians tried to prevent fever by continuous temperature monitoring of the patients who could not apply target temperature management for other reasons. A Levin tube was inserted as far as the stomach to provide early nutritional assistance to patients. For patient safety, enteral nutrition was given with the patient's head up and in a 45° angle. It was a continuous feeding method through the nasogastric pathway, and it was applied slowly at an injection rate of lower than 50 ml per hour using an infusion pump. The initial enteral feeding volume in the ICU was implemented within 100–150 ml per day. In addition, a slow infusion method was maintained for a few hours, rather than the bolus injection method [15,16]. At the time of hospitalization, information about the age, sex, weight, and height of the patients was recorded in the electronic medical record, and body mass index (BMI) was calculated based on these data. Moreover, information such as past medical history, first monitored rhythm, basic life support time, and advanced cardiovascular life support time was collected from relatives or paramedics. The length of hospital stay, type and frequency of complications, and neurologic prognosis were also collected from the medical records.
Study Outcomes
The primary outcomes were diet-related complications during intensive care, including aspiration pneumonia, gastric regurgitation, ileus, fever, hypoglycemia, and hyperglycemia after feeding. Aspiration pneumonia was defined as when gastric or pharyngeal substances entered the lower respiratory tract and adversely affected the lungs after resuscitation. The detection of aspiration pneumonia was based on anteroposterior chest X-ray and clinical signs of infection. Ileus was defined as a temporary pause or decrease of intestinal movement. The detection of ileus was based on anteroposterior abdominal radiography. Gastric regurgitation was defined as the reflux of gastric acid contents into the esophagus. Hypoglycemia was defined as a blood sugar level lower than 70 mg/dl, and hyperglycemia was defined as a blood sugar level higher than 200 mg/dl. Such complications, including gastric regurgitation, fever, and hypo/hyperglycemic events, were recorded by observation or opinions of the medical staff during intensive care. The secondary outcomes were survival rate and favorable neurologic status after post-cardiac arrest care. A favorable neurologic state was defined as a cerebral performance category (CPC) score of one or two points.
Statistical Analysis
Continuous variables are described as mean±standard deviation and were compared using the Mann-Whitney U-test based on the normality assumptions from the Kolmogorov-Smirnov test. Nominal data were calculated as a percentage of the frequency of occurrence and compared using the chi-square or Fisher’s exact test, as appropriate. The confounding variable was corrected by propensity score matching. The propensity score was estimated using a logistic regression model. Confusing variables in propensity score matching included sex, age, advanced cardiovascular life support time, targeted temperature management, and use of inotropics. The ENS (n=23) patients were matched 1:1 with DNS patients using greedy matching in caliper 0.2. After the propensity score matching, there was no significant difference in sex, age, advanced cardiovascular life support time, targeted temperature management, and use of inotropics between the groups. To compare the clinical complications between the ENS and DNS groups, a Poisson regression model was applied, and the relative risk and 95% confidence interval of each complication, survival rate, and neurologic prognosis were quantified. A P-value <0.05 was considered to indicate statistical significance. A certified statistician conducted all statistical analyses using SAS analytics software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline Characteristics
Between October 2019 and June 2020, 110 OHCA patients were treated in the emergency department. Sixteen patients aged <19 years or with non-medical etiologic cardiac arrest were excluded. In addition, seven patients were excluded because of inadequate information about enteral nutrition (Figure 1). Of the 87 patients, 39 were classified into the DNS group and 48 into the ENS group. Propensity score matching was used to select subjects with similar major characteristics between the two groups, and a total of 46 patients were enrolled.
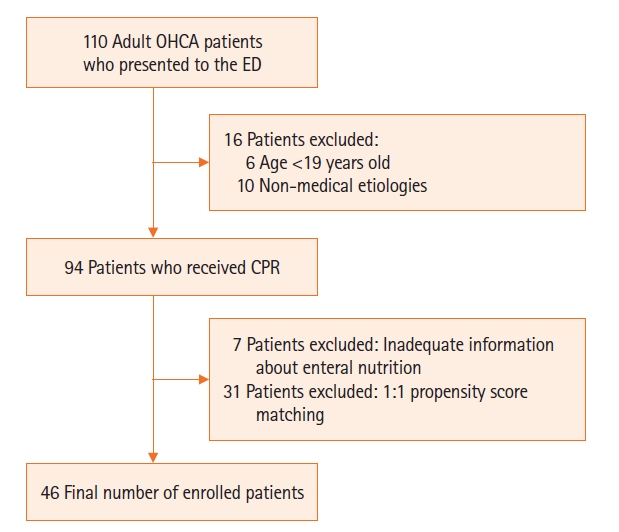
Study enrollment chart. OHCA: out-of-hospital cardiac arrest; ED: emergency department; CPR: cardiopulmonary resuscitation.
The mean age of the patients was 67.4 years, and 33 (71.7%) were men. The mean BMI was 22.6 kg/m2 and the Acute Physiology and Chronic Health Evaluation (APACHE) II score was 19.2. Patients had the following pre-arrest comorbidities: hypertension, 22 patients (47.8%); diabetes, 12 (26.1%); chronic kidney disease, 8 (17.4%); malignancy, 3 (6.5%); acute coronary syndrome, 4 (8.7%); chronic obstructive pulmonary disease, 3 (6.5%); cerebrovascular disease, 3 (6.5%); pulmonary thromboembolism, 1 (2.2%); and hyperlipidemia, 7 (15.2%). There was no difference in prior comorbidities between the two groups. There was no significant difference between initial rhythm (P=0.491), basic life support time (P=0.060), advanced cardiovascular life support time (P=0.888), inotropic use (P=1.000), and other laboratory findings. The mean feeding volume for the initial 5 days in the ENS group was significantly higher than in the DNS group (1,363.0 ml vs. 100.9 ml) (Table 1).
Primary Outcomes
The DNS and ENS groups had similar ICU hospitalization day stays (5.9 vs. 9.7, P=0.052). The APACHE II score was also statistically similar between the DNS and ENS groups (P=0.209). Regarding complications caused by nutrition support in the ENS group, aspiration pneumonia was present in four cases, gastric regurgitation in two, ileus in none, fever in 10, hypoglycemia in 8, and hyperglycemia in 11 cases. However, there was no significant difference in clinical complications between the ENS and DNS groups (Table 2).
Secondary Outcomes
The survival-to-discharge rate was higher in the ENS group than in the DNS group (86.9% vs. 39.1%, P<0.001). Among the CPC scores 1 and 2 between the ENS group and the DNS group, 13 patients (56.5%) were in the ENS group, and four patients (17.4%) were in the DNS group. There were significantly more patients with a good neurologic prognosis in the ENS group (Table 2).
Relative Risk of the Clinical Complications According to Timing of Enteral Nutrition
The researchers calculated relative risk by checking for the presence of complications according to the timing of enteral nutrition. Comparing the ENS group with the DNS group, the relative risk ratios for major complications were similar: aspiration pneumonia 0.95-fold, gastric regurgitation 1.09-fold, ileus 0.96-fold, fever 0.92-fold, hypoglycemia 1.20-fold, and hyperglycemia 1.17-fold. The occurrence of secondary outcome-related variables showed a higher relative ratio of 1.90 for good neurologic recovery and 4.67 for survival in the ENS group (Table 3). In addition, a comparative subgroup analysis was performed for 35 patients without initial shockable rhythms; there was also no significant difference in diet-related complications during post-resuscitation ICU care (Supplementary Tables 1-3).
DISCUSSION
This study retrospectively compared the occurrence of complications and prognosis following the implementation of early oral nutrition for 48 hours in patients who received post-cardiac arrest care. The main finding of the study is that ENS for resuscitated patients after propensity score matching had no significant difference in clinical complications compared to DNS. Considering the difference in the severity of patient condition and the short interval in enteral nutrition, it would be unreasonable to concede the higher survival rate or good neurologic outcome of the ENS group. However, it is a remarkable finding that the incidence of diet-related complications between the two groups was similar. In general, patients who were able to receive early enteral nutrition would be expected to have a relatively less severe condition. Nevertheless, it could be inferred that a similar incidence of diet-related complications in each group might occur at any time during the post-resuscitation period, regardless of the timing of enteral nutrition support.
Early application of enteral feeding within 24–48 hours in intensive care has been associated with a decrease in ICU stay and mortality among critically ill patients [17,18]. However, many critically ill patients are often exposed to malnutrition, which increases their mortality and morbidity [19-22]. According to the recent American Heart Association, European Cardiopulmonary Resuscitation Association, and the Korean Association of Cardiopulmonary Resuscitation guidelines for post-resuscitation cardiac arrest patients, there are some recommendations for specific nutritional supplement guidelines for these patients [2,23,24].
A literature review reveal opinions among experts based on the results of a few studies. Gutierrez et al. reported that in resuscitated patients after OHCA treated with extracorporeal cardiopulmonary resuscitation and targeted temperature management, delayed enteral nutrition was associated with improved neurologically favorable survival. They also found that adverse events related to enteral nutrition were not correlated with the timing of feeding initiation. However, the study involved obese patients with an average BMI ≥30 kg/m2, and the results may differ from those of patients with a normal BMI [25]. Martin et al. [24] reported a low incidence of respiratory complications and mortality rate despite prolonged morbidity and hospitalization after early enteral nutrition in patients with an average BMI of 28.5 kg/m2. Although there are differences in BMI between the patient groups, their results are similar to those of the present study regarding clinical complications and neurologic outcomes after intensive care .
Based on expert consensus, the 2016 SCCM and ASPEN guidelines recommend enteral nutrition should be withheld until patients are fully resuscitated or in a stable hemodynamic state [11]. Moreover, the European Society of Intensive Care Medicine recommended that during the initial target body temperature maintenance treatment, low-dose early enteral nutrition should be used, and then the feeding dose should be increased after rewarming [26]. In addition, they also recommended not delaying enteral nutrition even when neuromuscular blocking agents were used [26]. Therefore, although nutritional support has been planned for patients with spontaneous circulation recovery after cardiopulmonary resuscitation in the same way as for other intensive care patients, it seems necessary in the future to establish basic nutritional supplementation guidelines specifically for resuscitated patients based on related studies.
This study has several limitations. First, it was a retrospective, single-institution, and small-population study. Moreover, the difference was reduced by performing propensity score matching to ensure equivalence between two groups. However, in this process, the sample size is significantly reduced, which can be considered a weak point of this analysis. Therefore, it is difficult to generalize study results to other resuscitated patients after OHCA. Second, since this study was limited to enteral nutrition, the effect of parenteral nutrition on patients could not be found. Third, there is a possibility of changing the nutritional support method or feeding volume after the initial enteral support during intensive care. Thus, an exact quantitative causal relationship between nutritional support and clinical complications could not be determined. Fourth, the difference in mortality and neurologic prognosis confirmed by the difference in the timing of enteral nutrition may be the cause rather than the result; therefore, cautious interpretation is required. Fifth, the DNS group included some cases of early mortality and inability to provide enteral feeding during intensive care. Thus, the amount of enteral nutrition for the initial 5 days was much lower in the DNS group than in the ENS group. In conclusion, there was a similar incidence of diet-related complications during post-cardiac arrest care between the ENS and DNS groups.
KEY MESSAGES
▪ Supporting proper nutrition can reduce a patient's hospital stay and reduce patient morbidity.
▪ Early nutritional support for resuscitated patients demonstrated no significant difference in clinical complications when compared to delayed nutritional support.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: WJJ. Data curation: GWK. Formal analysis: JHH, YIR. Methodology: SOH. Project administration: KCC. Visualization: GWK. Writing–original draft: GWK. Writing–review & editing: WJJ.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2022.00696.
Baseline characteristics of the patients without shockable initial rhythm
acc-2022-00696-suppl1.pdfClinical outcomes according to timing of enteral nutrition in patients without shockable initial rhythm
acc-2022-00696-suppl2.pdfRelative risk analysis of early enteral nutrition compared to those of delayed enteral nutrition in patients with non-shockable initial rhythm
acc-2022-00696-suppl3.pdf