Clinical Application of the Quick Sepsis-Related Organ Failure Assessment Score at Intensive Care Unit Admission in Patients with Bacteremia: A Single-Center Experience of Korea
Article information
Abstract
Background
We evaluated the clinical usefulness of the quick Sepsis-Related Organ Failure Assessment (qSOFA) score (based on the 2016 definition of sepsis) at intensive care unit admission in Korean patients with bacteremia.
Methods
We retrospectively analyzed clinical data from 236 patients between March 2011 and February 2016. In addition to the qSOFA, the Modified Early Warning score (MEWS) and systemic inflammatory response syndrome (SIRS) criteria were calculated.
Results
The patients’ median age was 69 years, and 61.0% were male. Of the patients, 127 (53.8%) had a qSOFA score ≥2 points. They had significantly higher rates of septic shock, thrombocytopenia, and hyperlactatemia, and increased requirements for ventilator care, neuromuscular blocking agents, vasopressors, and hemodialysis within 72 hours after intensive care unit admission. They also had a significantly higher 28-day mortality rate. When analyzed using common thresholds (MEWS ≥5 and ≥2 SIRS criteria), patients with a MEWS ≥5 had the same results as those with a qSOFA score ≥2 (P < 0.05). However, patients with ≥2 SIRS criteria showed no significant differences.
Conclusions
Our results show that a qSOFA score ≥2 at admission is a useful screening tool for predicting disease severity and medical resource usage within 72 hours after admission, and for predicting 28-day mortality rates in patients with bacteremia. In addition, qSOFA scores may be more useful than SIRS criteria in terms of prognostic utility.
Introduction
Sepsis is a life-threatening organ dysfunction resulting from a dysregulated host response to infection and is the leading cause of morbidity and mortality worldwide [1,2]. Sepsis was defined by a consensus conference in 1991 [3]; however, the definition was revised in 2016 (sepsis-3) [2]. In sepsis-3, the quick Sepsis-Related Organ Failure Assessment (qSOFA) score was introduced as a means of screening for sepsis at the bedside based on a patient’s respiratory rate, blood pressure, and level of consciousness [2]. In addition, the recommendations suggest that patients with a qSOFA score ≥2 suspected to have an infection should be monitored closely.
Although several studies have suggested that compliance with the Surviving Sepsis Campaign bundles can benefit survival [4-7], compliance with resuscitation and management bundles is generally poor in many Asian intensive care units (ICUs) (including Korea) [8,9]. Moreover, there have been no multicenter studies regarding the current status of compliance with management recommendations at the national level because critical care resources and facilities at university hospitals in Korea are limited compared with Western countries [10].
Therefore, it is questionable whether qSOFA scores can be applied successfully in Korea. In addition, no largescale multicenter studies have reported the prognostic utility of this score. Also, the usefulness of the qSOFA score in Korea is unknown.
We hypothesized the qSOFA score would be useful in Korean patients. The present study investigated the clinical application and usefulness of the qSOFA score at ICU admission for predicting 28-day mortality in patients with a microbiologically diagnosed infection. In addition, we compared this score with other conventional early warning scores [3,11,12].
Materials and Methods
1) Study design and subjects
This retrospective study was conducted at a university-affiliated tertiary care hospital. This hospital has six functionally separate ICUs with 85 beds (medical, 12 beds; surgical, 10 beds; cardio-stroke, 14 beds; neurosurgical, 13 beds; emergency, 20 beds; and trauma, 16 beds) with full cardiovascular and close airway monitoring. All patients were managed according to therapeutic recommendations based on Surviving Sepsis guidelines and a lung-protective ventilator strategy [13,14].
We included patients who had various infectious causes with positive blood culture tests at ICU admission; all blood culture results were obtained within 3 days after ICU admission. The inclusion period was from March 2011 to February 2016. The exclusion criteria were patients younger than 18 years and those whose mental state could not be assessed. Also, patients who could not know 28-day mortality after ICU admission (example, transferred to other hospitals) were excluded. For all positive blood cultures, organism identification was performed by conventional and automated biochemical methods (VITEK 2; BioMérieux, Marcy l’Etoile, France) from March 2011 to February 2013, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonic, Bremen, Germany) from March 2013 to February 2016. The medical records and laboratory and radiological findings of all patients included in the study were reviewed. All investigators confirmed that the study objectives and procedures were complete, and they had full access to all data. The investigators completed a case report form for each patient; data were collected from September to December in 2016. This study was conducted with the approval of Institutional Review Board of Pusan National University Hospital (IRB No. 1612-003-049). This study had no impact on patient treatment.
2) Data collection
The following data were gathered from the medical records of each patient: age, sex, comorbidities before ICU admission, ICU admission route, and length of stay (LOS; ICU and hospital). The severity of illness was measured by the Acute Physiology and Chronic Health Evaluation (APACHE) II score, and accompanying organ failure was measured by the Sequential Organ Failure Assessment (SOFA) score [15,16]. APACHE II and SOFA scores were calculated using data from the first 24 hours of ICU admission.
The qSOFA was calculated at the time of ICU admission, which was defined as a systolic blood pressure ≤100 mmHg, respiratory rate ≥22 breaths per minute, and altered mental status (defined as a Glasgow Coma Scale score ≤13) [2]. To compare the prognostic utility with qSOFA, we also calculated the Modified Early Warning score (MEWS) at ICU admission and systemic inflammatory response syndrome (SIRS) criteria within the first 24 hours of ICU admission; these data were based on previously published definitions [3,11,12].
We also evaluated primary sources of infection at ICU admission, microbiological data (Gram staining, organism identification, and susceptibility testing), and the requirement for hemodialysis (defined as the use of any form of renal replacement therapy), neuromuscular blocking agents, vasopressors, and ventilator care within 3 days after ICU admission. In addition, the blood platelet count and arterial lactic acid level were determined during the first 3 days after ICU admission. Survivors were defined as patients that survived for 28 days after ICU admission.
3) Statistical analysis
Continuous variables are expressed as medians (interquartile range [IQR]) and categorical variables are expressed as numbers (percentages). Student t-test and the Mann-Whitney U-test were applied to compare continuous variables. The chi-square and Fisher exact tests (for small numbers) were used to compare categorical variables. To estimate predictive capabilities of the qSOFA score and other scores for our cohort, the receiver operating characteristic curves were used to determine cutoff value. Pearson correlation coefficients between the qSOFA score and MEWS and SIRS were calculated. Logistic regression analyses were performed to evaluate the qSOFA score as an independent prognostic factor in 28-day mortality. All statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). A two-tailed P-value <0.05 was considered to indicate significant difference.
Results
1) Baseline characteristics
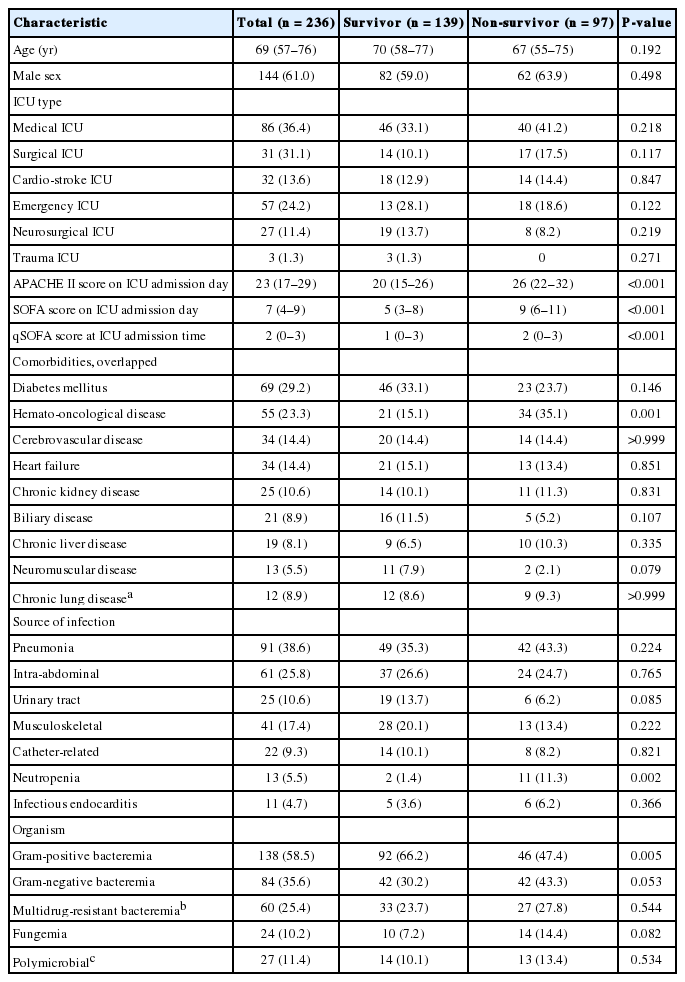
During the study period, we identified 236 patients with infectious causes that had positive blood cultures within 3 days after ICU admission. In the total patient population, 151 (64.0%) were admitted to the ICU via the emergency department (ED) and 144 patients (61.0%) received ventilator care during their ICU stay. The median ICU LOS and hospital LOS were 10 days (IQR, 5 to 19 days) and 25 days (IQR, 14 to 53 days), respectively. Diabetes mellitus was the most common underlying disease, and pneumonia was the most common source of bacteremia (Table 1). Gram-positive bacteria were the most commonly identified organisms (Table 1). Of total enrolled patients, 49 patients (20.8%) received surgical drainage aside from antibiotics. The clinical characteristics of all patients enrolled in this study and comparisons between survivors and non-survivors are presented in Table 1.
2) qSOFA score and patient outcomes
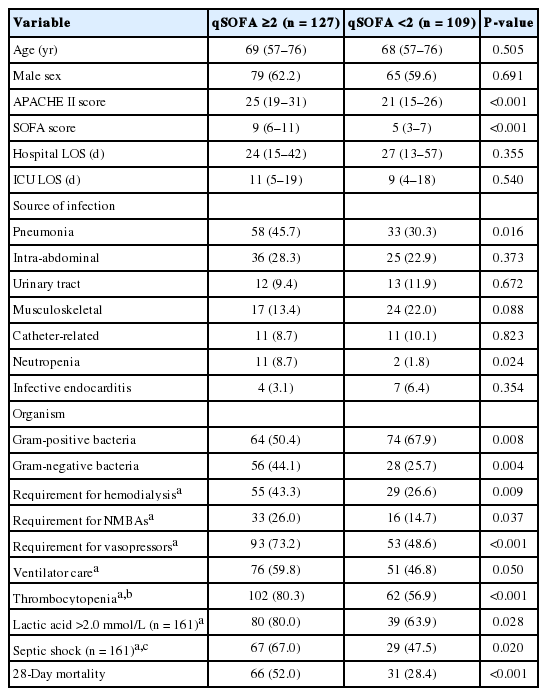
Figure 1 shows the number of patients for each qSOFA level and the corresponding mortality rates. Of the patients with a qSOFA score ≥2 (n = 127), 38 (29.9%) had all three criteria, followed by blood pressure and respiratory rate criteria (n = 35, 27.6%), mental status and blood pressure (n = 28, 22.0%), and mental status and respiratory rate criteria (n = 26, 20.5%). Patients with a qSOFA score ≥2 had significantly higher APACHE II and SOFA scores compared to those with a qSOFA score <2. In addition, these patients had significantly higher rates of septic shock, thrombocytopenia, and hyperlactatemia, and significantly greater requirements for ventilator care, neuromuscular blocking agents, vasopressors, and hemodialysis during the first 72 hours of ICU admission (Table 2). Further analysis indicated that patients with a qSOFA score ≥2 had significantly higher 28-day mortality rates than those with a qSOFA score <2 (Table 2). A univariate logistic regression analysis showed that a qSOFA score ≥2 was associated with 28-day mortality in our cohort (odds ratio, 2.722; 95% confidence interval, 1.582 to 4.683; P < 0.001).
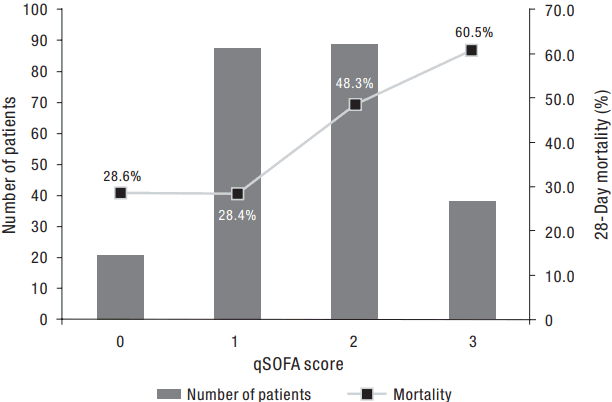
The number of patients for each qSOFA level (left Y axis) and the corresponding mortality (right Y axis). qSOFA: quick Sepsis-Related Organ Failure Assessment.
3) Comparison of qSOFA score with MEWS, SIRS, and SOFA
When we compared two conventional early warning scores (MEWS and SIRS), we found correlations between qSOFA score and MEWS (γ = 0.401, P < 0.001) and between qSOFA score and SIRS criteria (γ = 0.271, P < 0.001). Also, positive correlation was found between qSOFA score and SOFA score (γ = 0.465, P < 0.001).
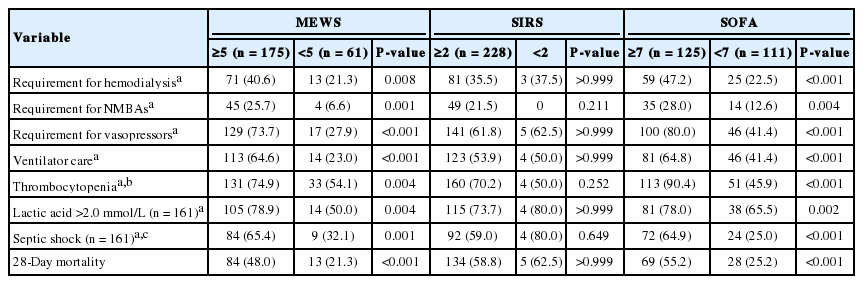
Further analysis using common thresholds for each conventional early warning score (MEWS ≥5, qSOFA ≥2, and SIRS criteria ≥2) according to published data was presented in Table 3 [3,11,17]. Patients with a MEWS ≥5 had significantly higher rates of septic shock, thrombocytopenia, and hyperlactatemia, and significantly greater requirements for ventilator care, neuromuscular blocking agents, vasopressors, and hemodialysis during the initial 72 hours after ICU admission than those of MEWS <5 (Table 3). In addition, they had higher 28-day mortality rates. However, patients with ≥2 SIRS criteria showed no significant differences compared to those with <2 SIRS criteria (Table 3). Also, we found the cutoff value of SOFA was 7, which were determined by receiver operating characteristic curves. When we compared between patients with a SOFA ≥7 and <7, patients with a SOFA ≥7 had same results as shown in patients with a MEWS ≥5 (Table 3).
Discussion
In the present study, we enrolled patients with bacteremia on ICU admission and evaluated the clinical utility of qSOFA scores at the time of ICU admission. In the present study, qSOFA score had positive correlation with SOFA score. Also, a qSOFA score ≥2 at ICU admission was associated with greater severity and higher medical resource use in the initial 72 hours after ICU admission. In addition, a qSOFA score ≥2 was a significant prognostic indicator for 28-day mortality. Although critical care resources are typically limited and there are distinct cultural differences compared to those in Western countries [9,10], our results suggested that a qSOFA score ≥2 at ICU admission would be a useful screening tool for predicting disease severity and mortality in patients with bacteremia.
After introducing the qSOFA score in sepsis-3 as a screening tool for organ dysfunction [2], comparisons of qSOFA score with some conventional early warning scores were reported [17-20]. In our study, the cutoff levels of MEWS and SIRS were used according to previous reported data [3,11,17]. Our results showed a MEWS ≥5 was associated with greater severity and higher medical resource use within 72 hours after ICU admission, and 28-day mortality after ICU admission, consistent with the observations associated with a qSOFA score ≥2. However, we found no prognostic utility of ≥2 SIRS criteria because 96.6% of the total patient population had ≥2 SIRS criteria (Table 3). Our findings suggest that qSOFA scores may be more useful than SIRS criteria as a prognostic indicator, consistent with a previous report [21]. In comparison with previous studies [17-20], however, our patients had bacteremia with a documented infectious focus at ICU admission, and they had a higher mortality rate. Therefore, additional large-scale studies including patients with non-bacteremia are required to compare qSOFA with other early warning scores as early screening tools.
In the present study, we found the survival rate was different according to admission route. In patients admitted to ICU via ED, there was no significant difference in the 28-day mortality rate between patients with qSOFA score ≥2 and <2 (37.5% vs. 29.1%, respectively; P = 0.274). In patients admitted from general wards, however, patients with qSOFA score ≥2 had significantly higher 28-day mortality rate than those with qSOFA score <2 (70.9% vs. 26.7%, respectively; P < 0.001). To find out these differences, we further evaluated where the patients were before being transferred to the emergency room of our hospital (i.e., home, heath care facility, or another teaching hospital), however, we could not investigate accurately because of the shortage of medical records. Therefore, further investigation is needed to evaluate the prognostic utility of qSOFA score for patients presenting to the ED.
Our study had several limitations. First, although qSOFA score was developed for patients with suspected infection presenting to the ED, in our study, we could not find the usefulness of qSOFA score for these patients due to the shortage of medical records. To assess the usefulness of this score, therefore, we enrolled patients who had documented infections with bacteremia admitted to ICU. Second, this study was conducted retrospectively; this may have resulted in information bias. Also, our enrolled patient populations were heterogeneous from six ICUs, which may be a bias. Third, our data represent the experience of a single center, so the results may not be representative of the general situation in Korea. Fourth, we expected that the qSOFA ≥2 score was associated with poor prognosis according to documented bacteria or sources of infection; however, we were unable to identify statistical significances in subgroup analysis due to the small sample size.
In conclusion, we investigated the prognostic utility of the qSOFA score at ICU admission for patients with bacteremia. Our results show that a qSOFA score ≥2 at admission could be useful as a screening tool for predicting clinical severity and medical resource use within 72 hours after admission, and for predicting the 28-day mortality rate. In addition, a comparison of qSOFA score with MEWS and SIRS criteria suggested that qSOFA scores are more useful than SIRS criteria. Prospective and large-scale studies are required to determine the prognostic utility of qSOFA scores in Korean ICUs.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. 2016R1C1B1008529).