The incidence of acute acalculous cholecystitis in critically ill COVID-19 patients
Article information
Dear Editor:
Coronavirus disease 2019 (COVID-19) can cause severe acute respiratory failure requiring admission to the intensive care unit (ICU). Over time, it has become clear that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) not only affects the respiratory system, but also has an impact, directly or indirectly, on many organs in the body, including the liver. Here we present three patients diagnosed with severe COVID-19 who developed acute acalculous cholecystitis (AAC) after a prolonged ICU stay.
AAC is a rare form of cholecystitis not associated with the presence of gallstones. In this case, inflammation of the gallbladder is due to hypomotility, which induces accumulation of bile with a secondary increase in intraluminal pressure that leads to inflammation, ischemia, and necrosis of the gallbladder wall [1,2]. The accumulation of bile can also promote bacterial colonization and sepsis. Numerous factors can contribute to the hypomotility that is the main stimulus that drives contraction and emptying of the gallbladder, including hemodynamic instability, dehydration, positive end-expiratory pressure in mechanically ventilated patients (by reducing hepatosplanchnic blood flow), opioid analgesics, sedation, and prolonged periods without enteral nutrition.
Between March 2020 and March 2021, 126 patients with COVID-19 were admitted to our ICU; of these, 96 required invasive mechanical ventilation (IMV). Three patients developed AAC. All these critically ill patients were included in a Spanish registry of COVID-19 patients, which was approved and exempted from the requirement for patient informed consent by Ethics Committee of our Hospital (123/2020).
The first case, a 73-year-old man, was admitted to the ICU for acute respiratory distress syndrome (ARDS) secondary to COVID-19. The patient required IMV for 34 days and enteral nutrition during the entire ICU stay. After 41 days in the ICU, the patient was discharged to the inpatient ward. Two days later, he developed abdominal pain and fever along with elevated C-reactive protein (CRP) levels. Abdominal ultrasound showed a hydropic gallbladder with thickened walls and incipient necrosis (Figure 1). Due to the patient’s frail condition, percutaneous drainage was performed, and antibiotics were administered. The patient responded well, and there was no need for cholecystectomy.
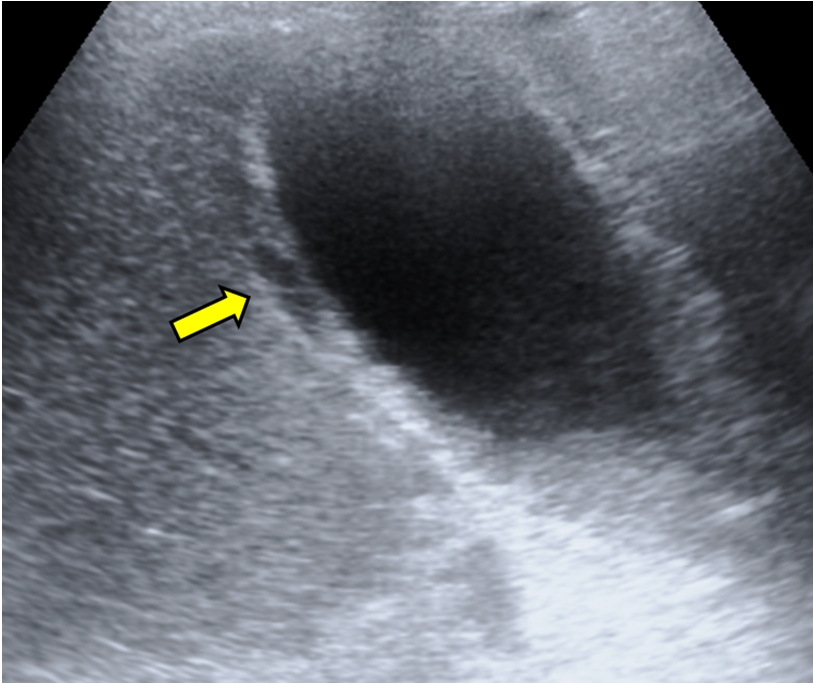
An ultrasound image suggestive of gangrenous cholecystitis. A hydropic gallbladder. Arrow: wall thickening (>5 mm) and necrosis.
The second patient, a 42-year-old man, was similarly admitted to the ICU for ARDS secondary to COVID-19. The patient required IMV for 35 days and received enteral nutrition throughout the ICU stay. After 36 days, he was discharged from the ICU to a rehabilitation center. Ten days later, the patient developed intermittent abdominal pain and was referred to the emergency department (ED) of our hospital. He presented with a slight but nonspecific increase in CRP levels and was transferred back to the rehabilitation center for continued care. Twelve days later, the patient reported persistent abdominal pain and was again referred to the ED. Abdominal computed tomography showed a distended gallbladder with poorly defined walls suggestive of edema (Figure 2). Laparoscopic cholecystectomy was performed, and antibiotics were administered. Staphylococcus warneri was isolated in the bile. The subsequent clinical course was good.
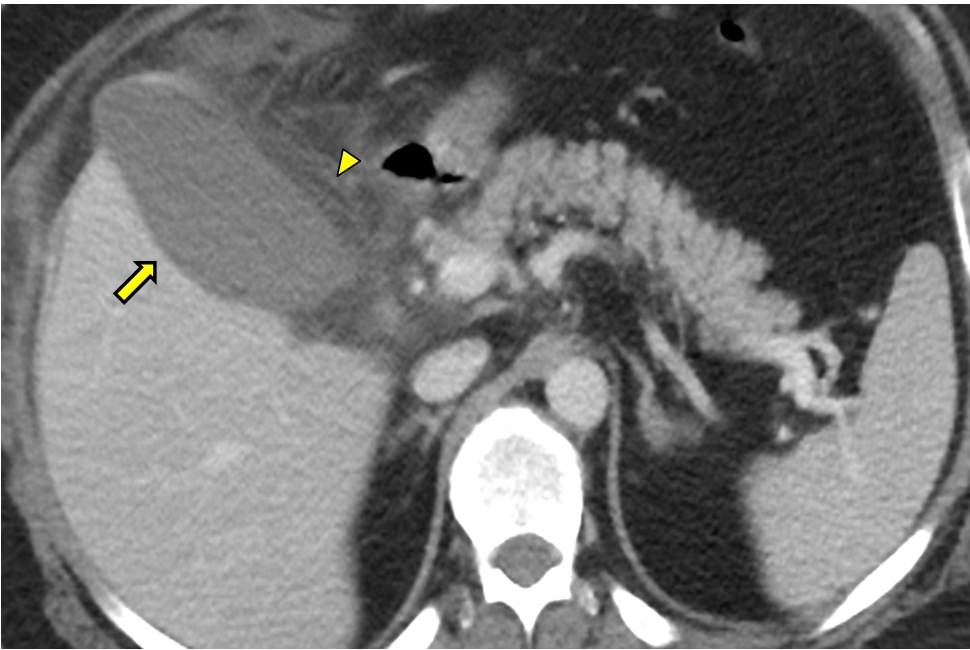
A computed tomography image suggestive of gangrenous cholecystitis. A hydropic gallbladder. Arrow: gallbladder wall showing decreased uptake that is poorly defined, which is suggestive of mural edema; Arrowhead: perivesicular inflammatory changes with trabeculation of the adjacent fat.
The third and final case, a 67-year-old man, was also admitted to the ICU for ARDS secondary to COVID-19. He required IMV for 63 days and received enteral nutrition throughout his ICU stay. After 70 days, he was discharged from the ICU to a rehabilitation center. Twenty days later, he developed abdominal pain, vomiting, and leukocytosis. An abdominal ultrasound revealed a distended gallbladder with an edematous wall. Laparoscopic cholecystectomy was performed, and antibiotics were administered. The patient required an emergency reoperation for hemorrhagic shock secondary to bleeding from the cystic artery. The subsequent clinical course was good.
During the COVID-19 pandemic, our strategy has been to provide protective ventilation with sedation, muscle relaxation, and pronation for at least the first 10 days after admission. Although this approach has led to good results regarding mortality (20% in patients on IMV), it also requires prolonged ICU stays (mean stay: 29±15 days, and 36, 41, and 70 day stays in these three cases) with high morbidity rates that are mainly attributable to myopathy and other “occult” conditions, such as AAC.
In critically ill patients, the incidence of AAC is low (<1%), although it can be as high as 5% in at-risk populations, such as those suffering from burns or trauma, and in patients undergoing major surgery [1,2]. The three cases described in this report represent approximately 3% of the COVID-19 patients who received IMV in our center (n=96) during this period, a proportion that is in line with the aforementioned incidence rate in at-risk populations. The diagnosis of AAC is difficult, more insidious and less severe than calculous cholecystitis, and requires substantial clinical expertise. It is important to emphasize that all three patients were diagnosed with AAC after discharge from the ICU (at 2, 12, and 20 days, respectively), which implies that at least one of these cases could have been diagnosed prior to ICU discharge.
There is little recently published literature on AAC in general critically ill patients, particularly in the context of COVID-19. Several case reports were published near the start of the pandemic that suggested a potential association between SARS-CoV-2 infection and cholecystitis, perhaps due to the cytokine storm or to the action of the virus itself on the angiotensin converting enzyme receptors in the gallbladder [3-5]. In our series however, the extended time interval from hospital admission to diagnosis (43, 48, and 90 days) leads us to believe that the association between the virus and AAC is more likely attributable to risk factors related to the extended ICU stay rather than to the virus itself.
We believe that the main value of the present scientific report is to highlight the presence of this “classic” but often overlooked condition in ICU patients. The low incidence and insidious clinical presentation of this disorder, coupled with the accumulated fatigue of hospital staff in the context of a long and exhausting pandemic, requires a high index of suspicion. To our knowledge, this is the first case series to report the incidence of AAC in this patient population.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
ACKNOWLEDGMENTS
None.
AUTHOR CONTRIBUTIONS
Conceptualization: DFL, FRC. Data curation: all authors. Formal analysis: DFL, FRC. Methodology: FRC. Project administration: FRC. Writing–original draft: FRC. Writing–review & editing: all authors.
