An unusual case of relapsing arrhythmia during veno-arterial extracorporeal membrane oxygenation cannulation
Article information
A 52-year-old woman presented after an out-of-hospital cardiac arrest with subsequent complications. Following successful primary percutaneous coronary intervention (PCI), she developed cardiogenic shock due to acute in-stent thrombosis. Despite an attempt to use an Impella CP device (Abiomed 14-Fr introducer), the patient worsened, leading to refractory pulseless ventricle tachycardia. Veno-arterial extracorporeal membrane oxygenation (ECMOLIFE, Eurosets) was initiated through left femoral artery (Bio-Medicus 19-Fr cannula) and right femoral vein cannulation (LivaNova bicaval RAP 23-25-Fr Can¬nula). A left femoral transvenous atrial balloon-septostomy (Ultraverse 5.0×40 mm) was performed after a failed attempt with the Impella CP device. A full-body computed tomography scan, part of the hospital protocol, revealed massive cerebral and intracardiac air emboli (Figure 1), prompting the initiation of comfort care.
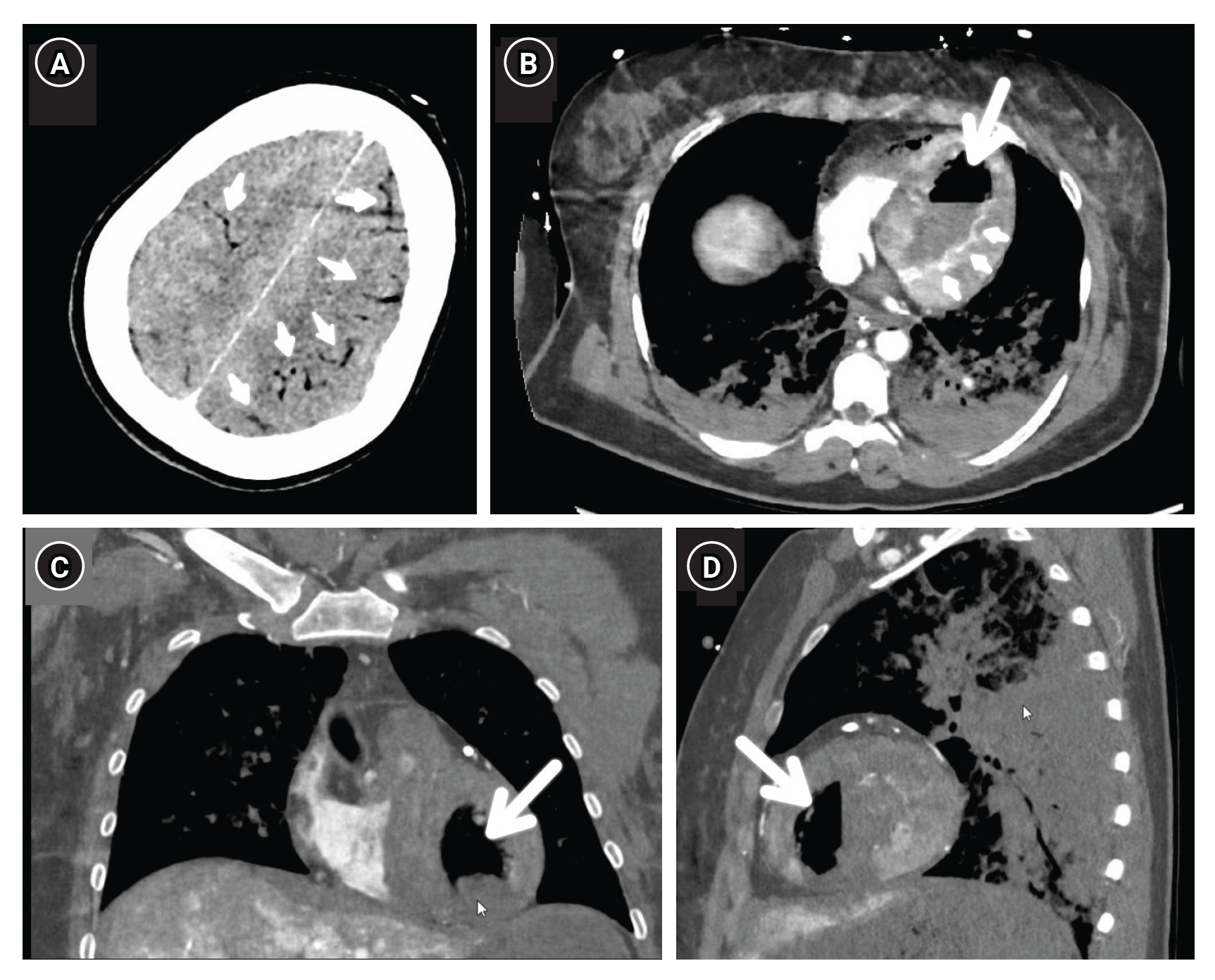
Computed tomography with contrast reveals overt cerebral air emboli (small arrows, A) as well as a massive air embolism (big arrows) in the left ventricle on axial (B), coronal (C) and sagittal (D) view (greyscale is equal to air in the lungs). Also note heterogeneous colouring of the left ventricular muscle (small arrows, B) due to recent infarction.
Notably, the fully functional bubble detector on the ECMO circuit did not trigger an alarm. The team attributed this complication to potential large-bore sheath manipulations in the left atrium or arterial system [1]. An alternative explanation considered was pulmonary vein damage during cardiopulmonary resuscitation, enabling air passage through a bronchovenous fistula into systemic circulation [2-4]. This case underscores the challenges and complexities in managing post-cardiac arrest scenarios and the importance of considering multiple factors in decision-making.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: RVL, TB, MM. Methodology: RVL, TB, MM. Formal analysis: RVL, TB. Data curation: RVL, TB, SL. Visualization: RVL. Project administration: RVL. Writing–original draft: RVL. Writing–review & editing: all authors.
ACKNOWLEDGMENTS
The Institutional Review Board has approved this study with exempt for informed consent.
