The impact of ketamine on outcomes in critically ill patients: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials
Article information
Abstract
Background
This meta-analysis aims to evaluate the effects of ketamine in critically ill intensive care unit (ICU) patients.
Methods
The search for randomized controlled trials (RCTs) in PubMed, Scopus, and the Cochrane Library was performed initially in January but was repeated in December 2023. Included studies compared ketamine with other traditional agents used in the ICU. We synthesized evidence using RevMan v5.4, presenting the results as forest plots, and used trial sequential analysis (TSA) software v. 0.9.5.10 Beta, presenting results as TSA plots. Our outcomes were mortality, pain, opioid and midazolam requirements, delirium rates, and ICU length of stay.
Results
Twelve RCTs involving 805 ICU patients (ketamine group, 398; control group, 407) were included in the meta-analysis. The ketamine group was not superior to the control group in terms of mortality, pain, mean and cumulative opioid consumption, midazolam consumption, and ICU length of stay. However, the model favored the ketamine group over the control group in delirium rate. This result is significant in terms of conventional boundaries (alpha=5%) but is not robust in TSA. The applicability of the findings is limited by the small number of patients pooled for each outcome.
Conclusions
No differences were found between ketamine and control groups regarding any outcome except delirium rate, where the model favored the ketamine group over the control group. However, this result is not robust as sensitivity analysis and trial sequential analysis suggest that more RCTs should be conducted in the future.
INTRODUCTION
Critical care is shifting toward reducing sedation and promoting early patient mobilization, highlighting effective pain management in critically ill patients. Poorly controlled pain in the intensive care unit (ICU) can worsen symptoms and conditions and may contribute to the development of long-term post-traumatic stress disorder [1]. While opioids remain a standard ICU treatment, they are associated with hypotension and potential for withdrawal or abuse. Moreover, opioids counterintuitively can increase pain, probably by altering the mechanisms of N-methyl-D-aspartate (NMDA) receptors [2]. Benzodiazepines, other frequently used sedatives, can cause excessive sedation leading to respiratory depression. Propofol and dexmedetomidine may be preferred to benzodiazepines due to fewer side effects and improved patient recovery, but they are limited by the risk of hypotension and high costs [3].
Ketamine is a promising alternative analgesic option for ICU patients. This phencyclidine derivative acts as a noncompetitive inhibitor of NMDA receptors [2]. Hypertension, tachycardia, delirium, and hallucinations are among the most common and concerning side effects of ketamine [4], and its hallucinatory effects can lead to potential abuse. However, in comparison with traditional ICU care, it has much lower risks of cardiovascular or respiratory depression [2]. Additionally, ketamine's fast onset of action and patient recovery make it an attractive analgosedative agent in critical care [3].
Existing research on ketamine in critical care includes two meta-analyses comparing several traditional ICU agents, including ketamine [1,5], a Cochrane systematic review focusing on postoperative patients [2], and a systematic review of randomized controlled trials (RCTs) and observational studies examining ketamine's impact on ICU patients [3]. Nevertheless, gaps in the literature remain, particularly concerning the optimal ketamine regimen for critically ill ICU patients [2].
In a mechanically ventilated (MV) ICU study [6], intravenous (IV) ketamine decreased the peak inspiratory pressure, improving analgesia and sedation. A 38-patient RCT suggested ketamine as a replacement for morphine for MV sedation [7]. A 469-patient RCT found ketamine to be a safe alternative to etomidate [8]. Ketamine demonstrated no harm to traumatic brain injury (TBI) patients in a shortcut review [9] and no difference in 30-day mortality rate compared to etomidate in a 2023 study [10].
Ketamine could be a good alternative to midazolam in "prehospital sedation of acutely agitated patients" [11]. Low-dose (60–120 µg/kg/hr) ketamine infusions, combined with opioids, may be used as a sedative-analgesic in the ICU [12]. In a 40-patient retrospective study [13], low-dose (5 µg/kg/min) ketamine reduced opioid consumption in MV adult patients. In cardiac surgery with cardiopulmonary bypass, ketamine reduces postoperative delirium in patients age >55 [14]. In a 55-patient RCT, a ketamine/propofol admixture (ketofol) was "associated with hemodynamic stability during the first 10 minutes after induction" [15].
Previous reviews indicated ketamine’s safety in severe TBI patients [16], potential efficacy in neurologically impaired patients during controlled ventilation [17], and no contradiction in TBI patients [18,19]. A 41-patient study [20] showed a decrease in the intracranial pressure and in use of vasopressors. However, there is a lack of high-level evidence on the effectiveness of ketamine in the ICU [21].
A 2019 meta-analysis on MV patients showed that ketamine reduced propofol dose (infusion rate) and ICU length of stay (LOS) and improved risk of delirium [22]. Similar results were observed in a double-blind RCT [23] in terms of mortality rates and ventilator-free days. One study reported reduced delirium with continuous infusion of low-dose (0.20 µg/kg/hr or 3.3 µg/kg/min) ketamine [23], while another did not observed such an effect (2018) [24], which led to further discussion on the effectiveness of ketamine [25,26].
A recent meta-analysis found no significant effect of ketamine on postoperative delirium, vasopressor requirement, and fentanyl consumption; however, hallucinations were more common in the ketamine group [27]. Postoperative delirium was not significantly affected by ketamine after general and regional anesthesia [28]. Therefore, our meta-analysis aims to better understand ketamine’s effect on important medical outcomes of mortality, pain intensity, mean and cumulative opioid consumption, midazolam consumption, delirium, and ICU LOS.
MATERIALS AND METHODS
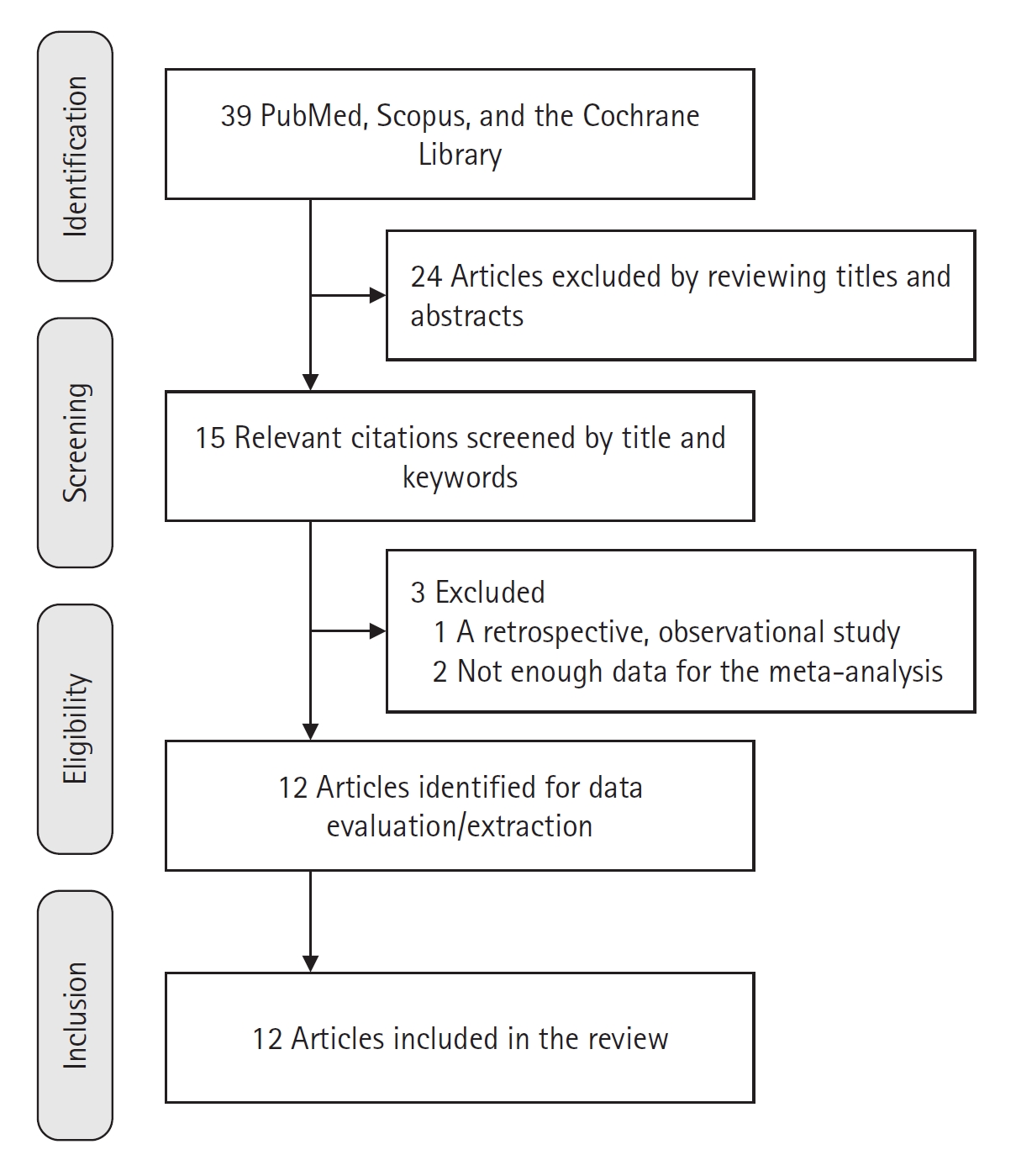
We sought RCTs in English that studied ketamine in critically ill ICU patients. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [29]. The search for relevant articles published before January 2023 was performed in PubMed, Scopus, and the Cochrane Library (Figure 1). The search was repeated in December 2023 and was conducted by one investigator. The following search terms or their combinations were used: "esketamine," "ketamine," "ketamin," "ketamines," "ketamines," "critical care," "critical," "care," OR "critical care," "critical," "care," "intensive," and "intensive care." We screened the titles and abstracts of the relevant studies against the inclusion criteria. Full-text articles of potentially eligible studies were assessed further for inclusion.
Participants and Population
Inclusion criteria—type of studies: RCTs only; patient age: no limitation; patient characteristics: critically ill patients; intervention: IV ketamine; language of publication: articles published in English. Exclusion criteria—non-RCT studies (e.g., technical reports, cadaver studies) that did not match our inclusion criteria. Outcomes—the primary outcome of our meta-analysis was the effect of ketamine on mortality. The secondary outcomes were pain intensity (visual analog scale, 0–10), consumption of opioids and midazolam, delirium rates, and length of ICU stay (days).
Data Extraction and Statistical Methods
We extracted the characteristics of studies in terms of population, intervention, comparison, and outcomes in the data table (Table 1). Data extraction was performed by one investigator using MS Word, while data aggregation was conducted by another investigator using MS Excel software (Microsoft). Data was analyzed by two investigators using the RevMan software and the trial sequential analysis (TSA) computer program (version 0.9.5.10 Beta; The Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Copenhagen University Hospital – Rigshospitalet, 2021 [30]). Within each synthesis group, we evaluated the homogeneity of the included studies by comparing their outcome measures according to our inclusion criteria. The studies were grouped based on reported outcomes. When necessary, data conversion were applied [31,32]. The following outcomes were included in the meta-analysis: mortality, pain intensity, mean opioid consumption, cumulative opioid consumption, midazolam consumption, delirium, and ICU LOS. The outcomes were carefully grouped by units and time points.
However, many other outcomes were not included due to insufficient data: mean daily ICP (mm Hg), mean daily sedation cost (U.S. dollars), mean duration of sedation (day), fluid dosage (ml), dopamine dosage (μg/kg/min), norepinephrine dosage (μg/kg/min), heart rate (beat/min), stroke volume index (ml/beat/m2), mean arterial pressure (mm Hg), mean pulmonary artery pressure (mm Hg), pulmonary capillary wedge pressure (mm Hg), time to extubation (day), incidence of side effects (nausea, hallucinations, hypoventilation, pruritus, seizure).
For synthesis groups with sufficient homogeneity, a random-effects meta-analysis was conducted using the Review Manager (RevMan) v.5.4 [33]. The outcomes were presented in forest plots. Mean difference (MD) or standardized mean difference (SMD) with 95% confidence interval (CI) were used for continuous outcomes. Dichotomous outcomes were described using risk ratio (RR) with 95% CI. Heterogeneity was estimated by the I2 statistic. We used subgroup analysis to explore possible causes of heterogeneity among study results. The sensitivity of the results was tested by excluding one study at a time, and results were reported if there were changes. For assessing reporting bias, funnel plots were used if there was a minimum of 10 studies available for each outcome.
For the trial sequential analysis, the risk of type I error (α) was 0.05, and that of type II error (β) was 0.20 when using a typical statistical power of 80% [34-38]. The relative risk reduction was estimated through analysis of low-bias risk trials [36,37] to exclude high-risk of bias studies that could potentially overestimate the intervention effect.
Risk of Bias Assessment
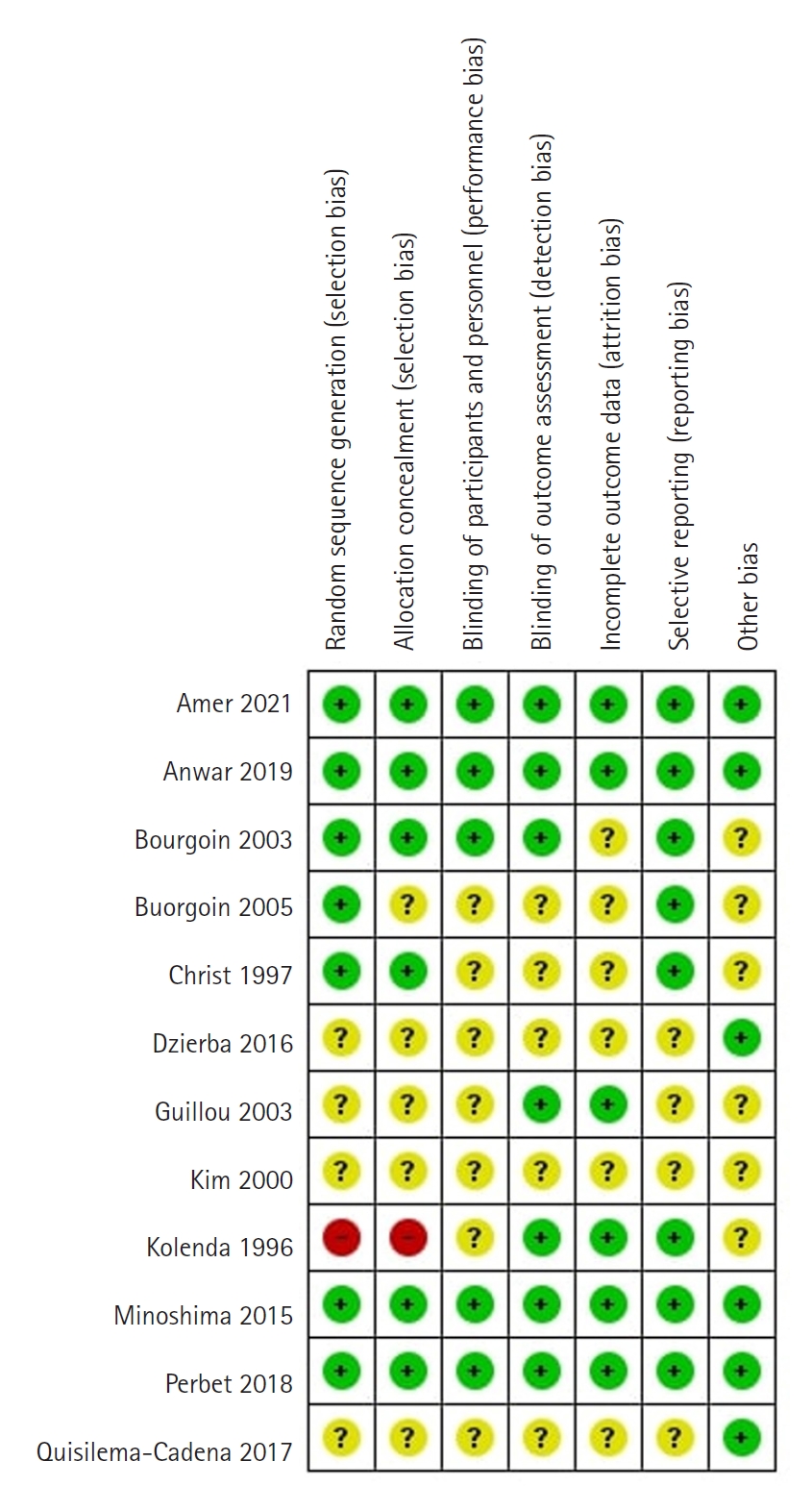
For the risk of bias assessment, we used the Cochrane RoB tool for RCTs [39]. This tool consists of seven domains to evaluate random sequence generation; allocation concealment; blinding of patients, staff, and investigators; handling of missing data; reporting of outcomes; and other sources of bias. In the "other" bias domain, we assessed information on funding. The overall risk of bias was assessed as "low," "unclear," or "high." The risk of bias summary was generated using RevMan 5.4.
RESULTS
We found 15 articles [7,23,40-52] that matched our search criteria (Figure 1). After further processing, 12 articles with 805 patients were selected for meta-analysis, with 398 patients in the ketamine group and 407 patients in the control group (Table 1) [7,23,40,41,43,45,46,48-52]. Included studies involved ICU patients suffering from neurological and cardiac diseases, some with acute respiratory distress syndrome or sepsis. The age of participants ranged from late teens to seventies. Procedures involved abdominal, cardiac, breast, orthopedic, and neurosurgeries. The duration of ketamine treatment ranged from 24 hours to 14 days. Some studies administered ketamine with pregabalin or midazolam. Ketamine was compared to a placebo or usual care in most studies but also with pregabalin, sufentanil with or without midazolam, and morphine.
Mortality
The model (Figure 2A) shows no significant difference in mortality between the groups (RR with 95% CI, 0.86 [0.63–1.16]; P=0.31, I2=0%). The result is not sensitive to the exclusion of any study. This result is verified by the TSA plot (Figure 2B) as the Z-curve is within the monitoring boundaries (“not statistically significant zone”).
Pain Intensity (0–10)
Figure 3 represents the pain intensity and shows no significant difference between the two groups (MD with 95% CI, –0.38 [–0.82 to 0.06]; P=0.09, I2=0%). A similar result was observed in the TSA model.
Mean Opioid Consumption (Standardized Units)
The model (Figure 4) for mean opioid consumption (in standardized units, i.e. in standard deviations) is based on two studies and does not favor either group (SMD with 95% CI, 1.61 [–2.73 to 5.95]; P=0.47, I2= 99%). The result is sensitive to exclusion of either study. The TSA model was not built for outcomes related to opioid consumption due to the use of SMD, which cannot be used in the current version of the TSA software.
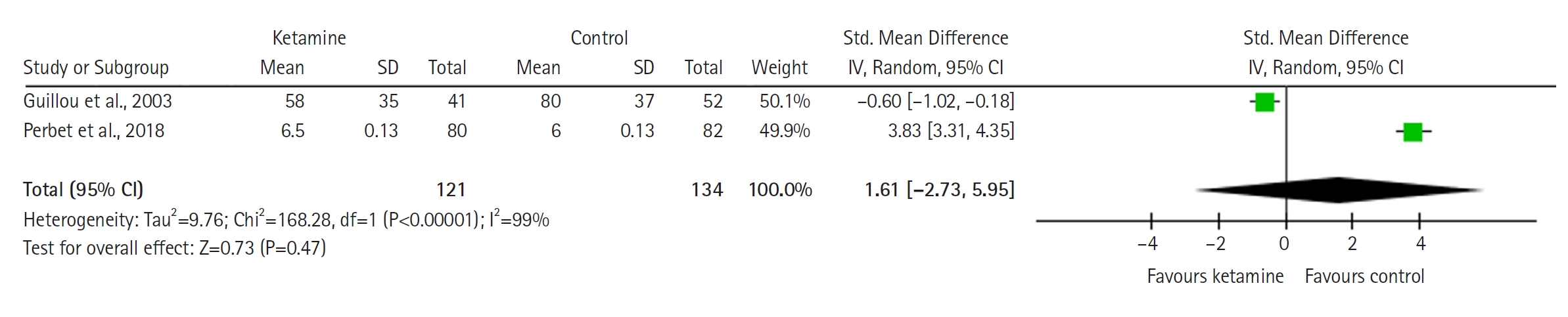
Forest plot of mean opioid consumption (standardized units). SD: standard deviation; Std: standardized; IV: inverse variance; CI: confidence interval.
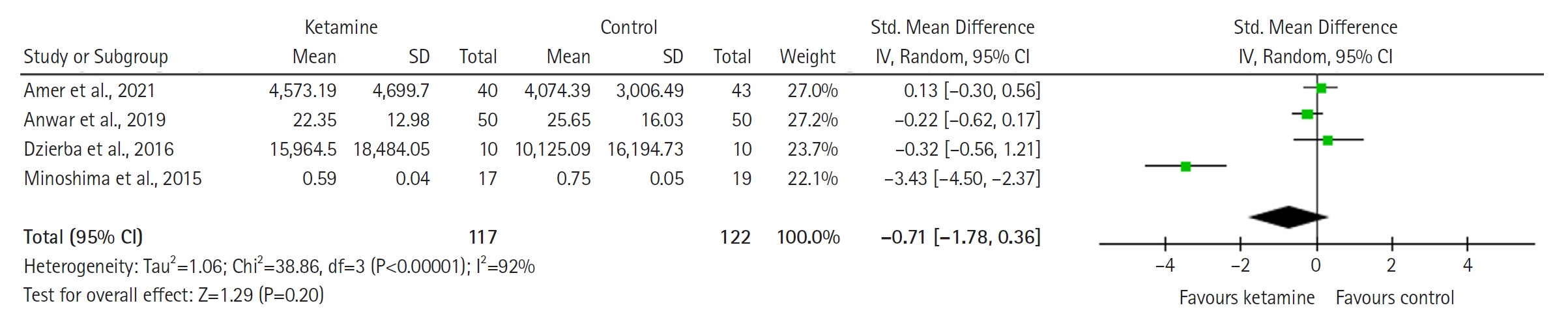
Cumulative Opioid Consumption (Standardized Units)
The forest plot (Figure 5) demonstrates cumulative opioid consumption and shows no significant difference between groups (SMD with 95% CI is –0.71 [–1.78 to 0.36]; P=0.20, I2= 92%).
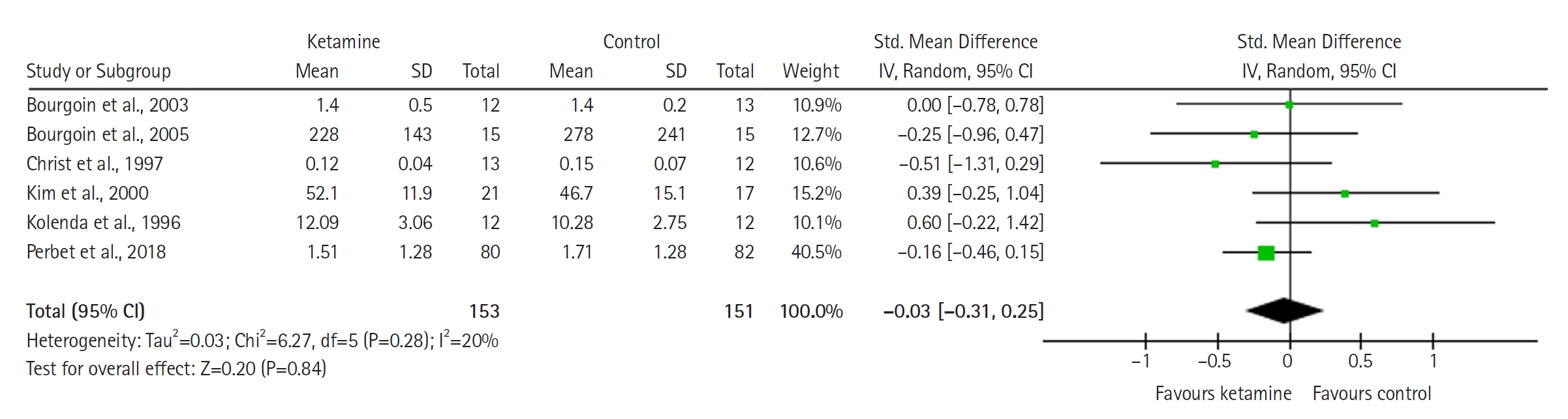
Midazolam Consumption (Standardized Units)
The model (Figure 6) shows no significant difference between the groups (SMD with 95% CI, –0.03 [–0.31 to 0.25]; P=0.84, I2= 20%).
Delirium
The model (Figure 7A) for delirium is based on four studies with a total number of 358 patients and favors the ketamine group (RR with 95% CI, 0.71 [0.51 to 0.99]; P=0.05, I2= 0%). The result is statistically significant and is sensitive to the exclusion of each study except that by Amer et al. [40]. The TSA plot (Figure 7B) confirms that the result is statistically significant in terms of conventional significance boundaries but not with TSA boundaries. The result favors ketamine and is significant in meta-analysis but not in TSA.
ICU LOS (Days)
The forest plot (Figure 8) represents ICU LOS, and does not favor either group (MD with 95% CI, 1.33 [–1.87 to 4.52]; P=0.42, I2= 0%). A similar result was observed in the TSA model.
Risk of Bias Assessment
We assessed four studies as having a low risk of bias (Figure 9) [23,40,41,49]. One study was a letter to the editor [50], and two studies described methods not in English [7,46], so it was difficult to assess all the bias domains in them. One study had a high risk of bias [45] because the randomization sequence was a patient number, which was not random. All other studies had an unclear risk of bias due to unreported information in some domains. Regarding random sequence generation, random tables and software for randomization were used. Sealed opaque envelopes or identical syringes and capsules were used for allocation concealment. Reporting bias was not assessed due to an insufficient number of studies, with fewer than 10 studies available for each outcome.
DISCUSSION
This meta-analysis of 12 RCTs demonstrated no difference between ketamine and control groups regarding mortality or medication use. Decrease in pain intensity did not reach clinical significance and was achieved in less than one of 10 patients. Ketamine caused less frequent delirium than placebo. At the same time, the length of ICU stay seemed to be at least one day longer in the intervention group. However, the results for this outcome did not reach statistical significance. These results can be explained by the varying severity of each patient's underlying medical condition and differences across the studies. Included studies were conducted in Europe, Asia, America, and the Middle East. The trials included mechanically ventilated patients with diverse conditions and ICU complications. Ketamine regimens and comparison groups also varied across the included studies. However, the applicability of the findings is limited by the risk of bias, a small number of patients pooled for each outcome, and potential language bias. The model for opioid consumption was highly heterogeneous, probably due to the differences in ketamine regimens and comparator groups across the included studies. Additional large RCTs are needed to determine the optimal regimen of ketamine for various patient populations.
The existing literature on ketamine in ICU patients suggests that it can decrease opioid consumption [1-3]. A network meta-analysis of various analgesics and sedatives found that ketamine did not reduce mortality compared to other approaches [5]. A Cochrane systematic review on postoperative pain management analyzed 130 studies that focused on various types of procedures and surgeries, including ENT (ear, nose and throat), thoracic, orthopedic, abdominal, urologic, and thyroid surgeries [2]. That review found that ketamine reduced postoperative pain intensity, as well as nausea and vomiting. In comparison with these reviews, our meta-analysis did not favor ketamine over the control groups in terms of pain intensity or opioid requirements. However, we did not find any differences in mortality between ketamine and control groups, similar to Wang et al. [5].
In this work, we evaluated the effect of ketamine in critically ill ICU patients. Our meta-analysis of 12 RCTs did not demonstrate differences between ketamine and control groups regarding mortality, pain intensity, opioid or midazolam consumption, and ICU LOS. However, ketamine reduced the delirium rate. This result is significant but may not be robust, and additional RCTs should be conducted in the future. The result on incidences of delirium was sensitive to exclusion of three of four studies, in which case the model showed no difference between the groups. The result on mean opioid consumption was sensitive to exclusion of either one of two studies. For all other outcomes, the result was not sensitive to exclusion of any study. Overall, the results of our study should be used with caution due to the limited number of studies included.
HIGHLIGHTS
▪ No difference in mortality was found between ketamine and control groups.
▪ Ketamine does not affect pain, intensive care unit length of stay, or analgesia use.
▪ Ketamine reduces delirium rate, but this needs to be confirmed by new randomized controlled trials.
▪ Conclusions are limited by the risk of bias.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This meta-analysis was supported by the Nazarbayev University Faculty Development Competitive Research (grants no. 11022021FD2906 and SOM2024005).
ACKNOWLEDGMENTS
None.
AUTHOR CONTRIBUTIONS
Conceptualization: DV. Methodology: YA, DV, KT. Supervision: DV. Funding: YA, DV. Validation: YA, DV. Data curation: AN, YA. Writing–original draft: all authors. Writing–review & editing: all authors.