Abstract
-
Background
- Pulmonary complications including pneumonia and pulmonary edema frequently develop in critically ill surgical patients. Lung ultrasound (LUS) is increasingly used as a powerful diagnostic tool for pulmonary complications. The purpose of this study was to report how LUS is used in a surgical intensive care unit (ICU).
-
Methods
- This study retrospectively reviewed the medical records of 67 patients who underwent LUS in surgical ICU between May 2016 and December 2016.
-
Results
- The indication for LUS included hypoxemia (n = 44, 65.7%), abnormal chest radiographs without hypoxemia (n = 17, 25.4%), fever without both hypoxemia and abnormal chest radiographs (n = 4, 6.0%), and difficult weaning (n = 2, 3.0%). Among 67 patients, 55 patients were diagnosed with pulmonary edema (n = 27, 41.8%), pneumonia (n = 20, 29.9%), diffuse interstitial pattern with anterior consolidation (n = 6, 10.9%), pneumothorax with effusion (n = 1, 1.5%), and diaphragm dysfunction (n = 1, 1.5%), respectively, via LUS. LUS results did not indicate lung complications for 12 patients. Based on the location of space opacification on the chest radiographs, among 45 patients with bilateral abnormality and normal findings, three (6.7%) and two (4.4%) patients were finally diagnosed with pneumonia and atelectasis, respectively. Furthermore, among 34 patients with unilateral abnormality and normal findings, two patients (5.9%) were finally diagnosed with pulmonary edema. There were 27 patients who were initially diagnosed with pulmonary edema via LUS. This diagnosis was later confirmed by other tests. There were 20 patients who were initially diagnosed with pneumonia via LUS. Among them, 16 and 4 patients were finally diagnosed with pneumonia and atelectasis, respectively.
-
Conclusions
- LUS is useful to detect pulmonary complications including pulmonary edema and pneumonia in surgically ill patients.
-
Keywords: complication; lung; surgical intensive care; ultrasonography
Introduction
Critically ill patients in surgical intensive care units (ICUs) have been severely injured, present with acute surgical emergencies, require prolonged and complex elective surgical procedures, or have severe underlying medical conditions [1]. They have risks for developing all of the potential problems that afflict nonsurgical patients in ICU and also have risks for complications related to surgical procedures [2]. Pulmonary complications, such as atelectasis, pneumonia, and pulmonary edema, which are associated with in-hospital mortality and length of hospital stay, frequently develop in critically ill surgical patients [1,2]. Although portable chest radiography and physical examination of the respiratory system are routinely performed for most patients in surgical ICUs, the application of chest radiography in the ICU for the detection of pulmonary complications is limited because of low diagnostic yields [3,4].
Lung ultrasound (LUS) has emerged in recent years as a bedside noninvasive test [5-8]. The use of bedside LUS in the ICU is increasing due to its ease of use, accessibility, safety profile, and immediate feedback [6-8]. Previous studies showed that LUS is useful for accurate diagnosis of various anatomical abnormalities including pleural effusion, diffuse interstitial syndrome, pneumothorax, pulmonary consolidation, and pulmonary abscess [5-12]. Furthermore, previous studies focused on comparing LUS with chest radiography and showed LUS had a high degree of diagnostic accuracy [8,12-14]. While many studies focused on diagnostic performance of LUS for patients in ICUs, none of the studies have provided insight into how to actually use LUS in a surgical ICU. The objective of this study was thus to investigate the clinical use of performing LUS in a surgical ICU.
Materials and Methods
1) Patients
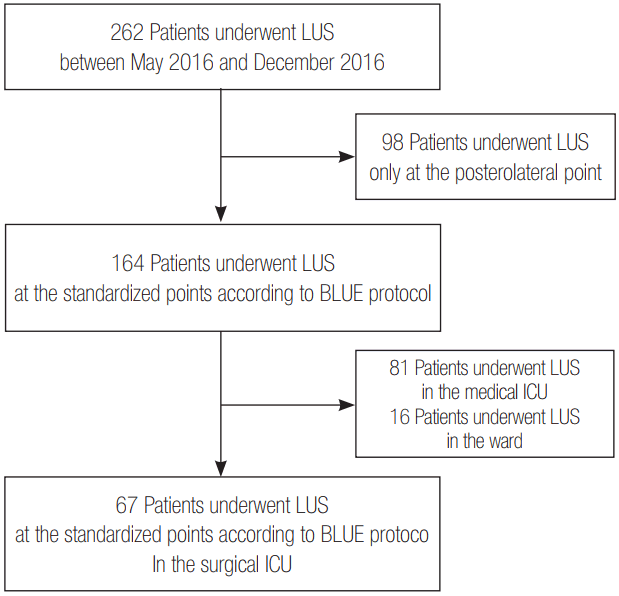
We retrospectively reviewed the medical records of 262 patients who had previously undergone LUS at Inje University Ilsan Paik Hospital between May 2016 and December 2016. After excluding 98 patients who underwent LUS only at the posterolateral point for identification of pleural effusion, we performed LUS on 164 patients at standardized points according to bedside LUS in emergency (BLUE) protocol [7]. Among them, 67 patients who underwent LUS in the surgical ICU were enrolled in this study, excluding 97 patients who underwent LUS in the medical ICU (n = 81) and the ward (n = 16) (Figure 1).
The Institutional Review Board of Inje University Ilsan Paik Hospital approved this study, including the review and publishing of information obtained from patient records (IRB No. 2016-12-023). The requirement for informed consent was waived for the use of patient medical data because all personally identifying information was removed before analysis.
2) Measurement
Patient medical records were reviewed to obtain data on demographic features, comorbidities, medical conditions, symptoms, laboratory data, radiologic findings, and LUS findings. Comorbidities including coronary artery disease, atrial fibrillation, chronic obstructive lung disease, rib fracture, chronic kidney disease, and underlying malignancy as well as whether patients underwent lung surgery previously were reviewed. Arterial blood gas analysis was performed for all patients undergoing LUS.
To diagnose and manage pulmonary complications, all patients consulted with one physician (HKK), intensivist, and pulmonologist. They were selected for LUS based on clinical needs for diagnosis of pulmonary complications, which occurred during the intensive care period in surgical ICU. Indications for LUS included hypoxemia, abnormal chest radiographs without hypoxemia, fever without both hypoxemia and abnormal chest radiographs, and difficult weaning. Difficult weaning is identified for patients in two situations, i.e., if the patient fails initial weaning and requires up to three spontaneous breathing tests (SBTs) or if the patient requires up to 7 days from the first SBT to achieve successful weaning [15].
Chest radiography was performed in all study patients in the morning prior to receiving LUS. All chest radiographs were obtained in the anteroposterior view using mobile equipment. Chest radiograph findings were divided into three classifications including unilateral air space opacification, bilateral air space opacification, and no significant abnormality.
The final diagnosis was formulated by two pulmonologists, who reviewed the clinical manifestations, radiologic findings, laboratory data, LUS findings, and clinical progression.
3) Lung ultrasound measurements
LUS was performed with the obtained clinical information including symptoms, laboratory data, and chest radiographs. All LUS examinations were performed by two well-trained experts (HKK and HJS) with a 13-MHz linear and 5-MHz curved array probe place over six standardized points for the BLUE protocol using an Acuson X300 ultrasound system (Siemens Healthineers, Erlangen, Germany) [7]. For patients who experienced difficult weaning during SBT [16], diaphragmatic movement for inspiration and expiration was additionally measured at one of the lower intercostal spaces in the right anterior axillary line for the right diaphragm and the left midaxillary line for the left diaphragm. Ultrasonographic diaphragmatic dysfunction was diagnosed if an excursion was <10 mm or negative.
According to the BLUE protocol, at the four anterior chest walls, lung sliding with predominant A-lines, bilateral lung rockets, anterior lung rockets associated with abolished lung sliding, and unilateral lung rockets defined the A-profile, the B-profile, the B’-profile, and the A/B profile, respectively [7]. Anterior lung consolidation, regardless of number and size, defined the C-profile. At the two posterior chest walls, lung consolidations and pleural effusions are evaluated [7,11-13].
In LUS, pulmonary edema was defined as the presence of the B-profile with or without bilateral pleural effusion [7]. Pneumonia was defined as the presence of the B’- profile, the A/B profile, the C-profile, and/or lung consolidations regardless of unilateral effusion at posterior chest wall [7,10-12,17]. Diffuse interstitial pattern with anterior consolidation was defined as the presence of the B-profile and the C-profile at least one anterior chest wall (Table 1). Pneumothorax was approached in LUS using the sole abolition of lung sliding [18]. Pleural effusion was defined as the presence of an anechoic space between the parietal and visceral pleura [12].
A total of 107 LUS examinations were performed in 67 patients. When subjects underwent more than one LUS examination during the study period, data from only the first examination were used in the analysis.
4) Statistical analysis
Baseline characteristics are presented as medians and interquartile ranges (IQRs) for continuous variables and as numbers (%) for categorical variables. The data were compared using the Mann-Whitney U-test for continuous variables and Pearson’s chi-square test or Fisher exact test for categorical variables. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
1) Baseline characteristics
The baseline characteristics of 67 patients are summarized in Table 2. The study patients included 47 males (70.1%) and 20 females (29.9%), with a median age of 68 years (IQR, 55 to 78 years). The median number of examined LUS per person during the study period was 1 (IQR, 1 to 2). Of the 67 patients, 33 (49.3%), 15 (22.4%), 10 (15.0%), 6 (9.0%), 2 (3.0%), and 1 (1.5%) were neurosurgical, thoracic surgical, orthopedic surgical, abdominal surgical, obstetric surgical, and head and neck surgical patients, respectively. Twenty-three patients (34.3%) had trauma. Routine surgical procedures were performed for 49 patients (73.1%).
2) Indications and findings of performed LUS
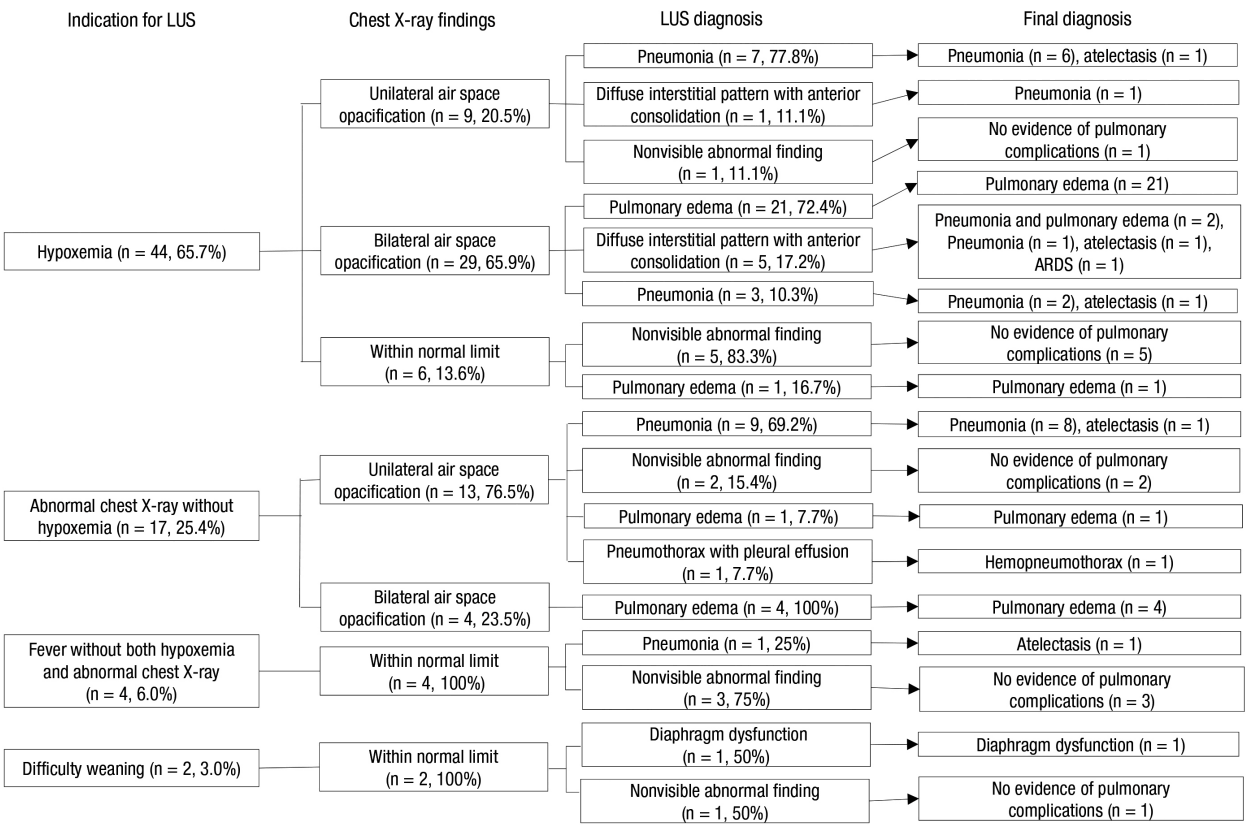
The indication for LUS included hypoxemia (n = 44, 65.7%), abnormal chest radiographs without hypoxemia (n = 17, 25.4%), fever without both hypoxemia and abnormal chest radiographs (n = 4, 6.0%), and difficult weaning (n = 2, 3.0%) (Figure 2).
A total of 55 patients were diagnosed with pulmonary edema (n = 27, 41.8%), pneumonia (n = 20, 29.9%), diffuse interstitial pattern with anterior consolidation (n = 6, 10.9%), pneumothorax with effusion (n = 1, 1.5%), and diaphragm dysfunction (n = 1, 1.5%), respectively, via LUS. Among 27 patients who were diagnosed with pulmonary edema via LUS, 21 (77.8%) had bilateral pleural effusion. Among 20 patients who were diagnosed with pneumonia by LUS, both the A/B-profile and C-profile were observed in 16 patients (80%) (Table 3).
One patient, who was confirmed to have a pneumothorax with effusion via LUS, was diagnosed with hemopneumothorax using pleural fluid analysis. For 12 patients, LUS did not identify any lung complications. Among six patients who had hypoxemia, three patients had difficulty in self-expectoration of secretion, and three patients were diagnosed with sepsis. One patient, who had an abnormal chest radiograph without hypoxemia, was not finally diagnosed with pulmonary complications. Among patients who had fever without both hypoxemia and abnormal chest radiographs, two patients had central fever after cerebral hemorrhage, and one patient had a deep neck infection. One patient with difficult weaning had cardiac load caused by systolic and diastolic dysfunction.
3) Final diagnosis based on the location of space opacification on chest radiographs and LUS findings
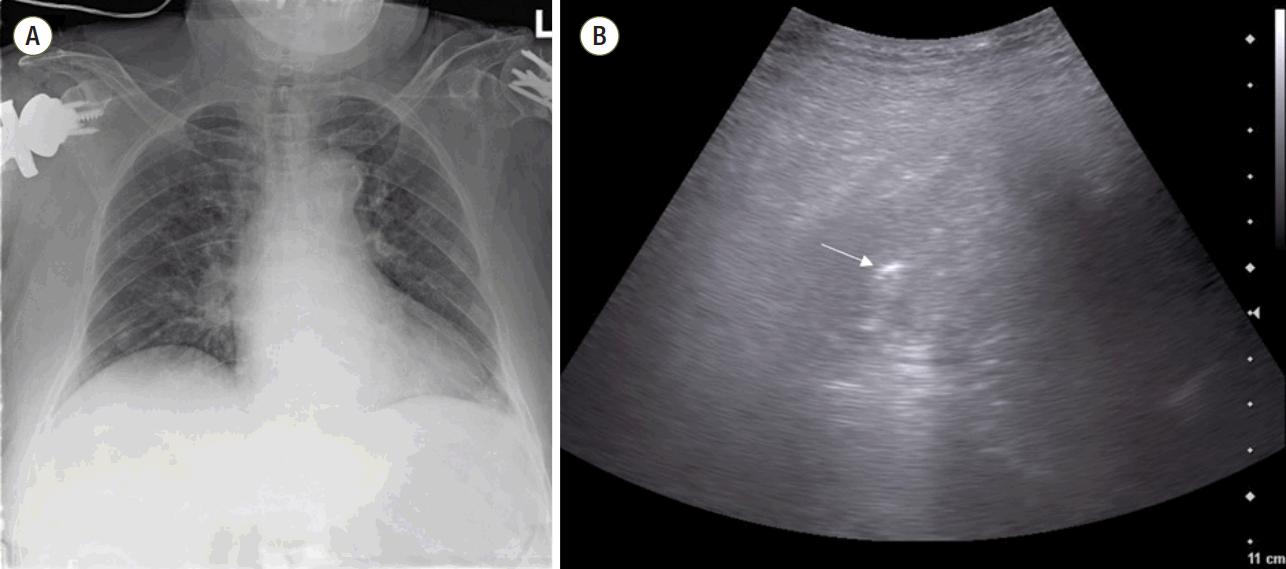
Based on the chest radiography findings, patients had unilateral air space opacification (n = 22, 32.8%), bilateral air space opacification (n = 33, 49.3%), and no abnormal findings (n = 12, 17.9%). Among 22 patients with unilateral air space opacification, one patient (4.5%) was finally diagnosed with pulmonary edema, and three patients (13.6%) were not diagnosed with any pulmonary complications. Among 33 patients with bilateral air space opacification, five patients (15.2%) were diagnosed with pneumonia regardless of pulmonary edema. Further, of 12 patients without chest radiograph abnormalities, three patients (25%) were diagnosed with pulmonary complications (Table 4). The image comparing chest radiograph and LUS in one case among three patients is shown in Figure 3.
Based on LUS diagnosis, all 27 patients with pulmonary edema for LUS were finally diagnosed with pulmonary edema. Among 20 patients with pneumonia for LUS, 16 patients (80%) and four patients (20%) were finally diagnosed with pneumonia and atelectasis, respectively. The 12 patients whose LUS abnormal findings were inconclusive were not diagnosed with any pulmonary complications (Table 5).
Discussion
In the present study, we showed LUS was used for evaluation of hypoxemia, abnormal chest radiograph findings without hypoxemia, fever, and difficult weaning in 67 surgically ill patients. LUS was helpful for diagnosis of pneumonia, atelectasis, pulmonary edema, or a combination of these diseases in 53 patients (79.2%). In addition, based on the location of space opacification on chest radiographs, LUS was used to diagnose pneumonia or atelectasis in four patients (17.4%) with bilateral abnormality and normal findings, and pulmonary edema in two patients (12.8%) with unilateral abnormality and normal finding.
The presence of infiltrates on a chest radiograph is considered the definitive marker for diagnosing pneumonia when clinical and microbiologic features are supportive [19-21]. Lung infiltrates may be difficult to identify in critically ill patients for whom only portable chest radiography is available [20]. Furthermore, clinical symptoms including fever, cough, and sputum production may be difficult to identify in critically ill surgical patients. Decreased mental status in ICU patients makes it hard to express symptoms of pneumonia [1,2]. Moreover, both hypothermia and unreliable temperature due to intervention such as renal replacement therapy could mask fever [22,23]. Previous studies showed that the sensitivity and specificity for the diagnosis of pneumonia using LUS were 81%–97% and 88%–97%, respectively, suggesting that LUS is a useful test for diagnosing pneumonia when chest radiographic results are negative or inconclusive [13,14,24-28]. In this study, LUS detected lung parenchymal consolidation with air bronchogram in one patient without chest radiograph abnormalities.
Pulmonary edema is secondary to accumulation of fluid in the lung interstitium or alveolar space [29]. Patients with extensive traumatic or surgical tissue injury, critical illness, or sepsis require replacement fluid therapy in addition to maintenance therapy, but fluid accumulation leading to a positive fluid balance could increase pulmonary edema in critically ill surgical patients [30]. Although chest radiographic findings of pulmonary edema include diffuse infiltrates regardless of pleural effusion and bilateral alveolar filling pattern [29], portable chest radiography in the critical care setting often yields inaccurate images [31,32]. Previous studies showed the positive relationship between diffuse lung interstitial involvement and bilateral sonographic B-lines [31,33-35]. In this study, two patients who showed no suspicious symptoms of pulmonary edema on chest radiographs were diagnosed with pulmonary edema during LUS. Additionally, noncardiogenic pulmonary edema is associated primarily with other clinical disorders, including pneumonia [29]. The final diagnosis for two patients in this study was pulmonary edema and pneumonia.
The usefulness of LUS for diagnosis of pleural effusions has been previously demonstrated by several studies that performed LUS for ICU patients [32,36-38]. LUS helps distinguish between effusion and lung consolidations and is reliable for the identification of pleural effusion when compared to portable chest radiography [32,37]. The appearance of pleural effusion on LUS scans can suggest the nature of the fluid although a definitive diagnosis requires a thoracentesis to allow biochemical and microbiological analyses [39]. In addition, the ultrasound feature of multiple comet-tail artifacts can be helpful in the diagnosis of alveolar-interstitial syndrome [33]. Therefore, LUS could be useful to distinguish between pneumonia and pleural effusion.
Difficult weaning from mechanical ventilation is associated with increased patient morbidity and mortality [15]. Various factors including respiratory load, cardiac load, neuromuscular abnormalities, and metabolic disorders could make it difficult to wean from mechanical ventilation [15]. LUS can be used to find diaphragmatic dysfunction caused by critical illness or neuromuscular abnormalities [16,40,41] and helps diagnose pneumonia or pulmonary edema related to respiratory load [12]. In our study, LUS was performed for detecting causes of difficult weaning in two patients. Since LUS can be used to assess lung parenchyma, pleural space, and diaphragm, its use may be helpful to detect causes of difficult weaning.
In surgical ICUs, the detection of pulmonary complications, which are major causes of morbidity and mortality, is an important challenge [1]. In our study, LUS was useful for diagnosing pulmonary complications, such as pneumonia, pulmonary edema, and diaphragm dysfunction. Especially, all patients who had visible abnormal findings during LUS were not diagnosed with pulmonary complications, suggesting that LUS could be used to exclude pulmonary complications.
There are several limitations to the present study. First, this study was conducted as a retrospective design in a single center ICU. Patients admitted to surgical ICU could vary widely by institution. Second, there are operator- dependent limitations to LUS. LUS was performed by two LUS experts. However, to reduce operator-dependent limitations, all patients’ ultrasonographic findings were reviewed by a supervisor. Third, since color Doppler in lung consolidation was not performed, we might not distinguish between dependent atelectasis and consolidation of infectious nature using LUS in several mechanically ventilated patients. The vascular pattern indicators within the consolidation, as assessed by color Doppler, could determine the etiology of pulmonary consolidations [24]. However, clinical findings including patient history, physical examination, and laboratory analysis help distinguish between atelectasis and pneumonia in the present study. Fourth, many patients who underwent trauma and thoracic surgery were enrolled in this study. Lung contusion associated with severe trauma (Injury Severity score ≥16 and Glasgow Coma scale <8) is difficult to distinguish from pneumonia via LUS [42]. However, we did not use LUS as a tool for distinguishing lung contusion from pneumonia in this study because most patients with severe trauma had undergone chest computed tomography during ICU admission periods. Performing LUS in patients who underwent thoracic surgery could be negatively affected by various factors such as anatomical change due to manipulation, surgical dressings, and chest tube. Many patients were diagnosed with pulmonary complications on the opposite side of their thoracic surgery in this study. Nonetheless, LUS in patients with thoracic surgery may have many limitations.
In conclusion, LUS is used to evaluate clinical needs including hypoxemia, fever, abnormal chest radiographic findings, and difficult weaning. Furthermore, it is useful for diagnosis of pulmonary complications, such as pneumonia, pulmonary edema, and diaphragm dysfunction. Therefore, it is a useful method for evaluating surgically ill patients who might have pulmonary complications.
NOTES
-
No potential conflict of interest relevant to this article was reported.
Figure 1.Flow chart for study enrollment. LUS: lung ultrasound; BLUE: bedside LUS in emergency; ICU: intensive care unit.
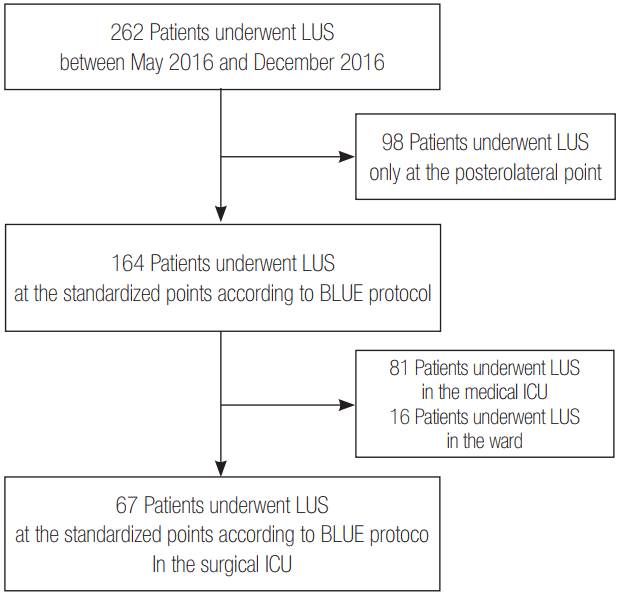
Figure 2.Overall flow diagram outlining the diagnoses of pulmonary complications. LUS: lung ultrasound; ARDS: acute respiratory distress syndrome.
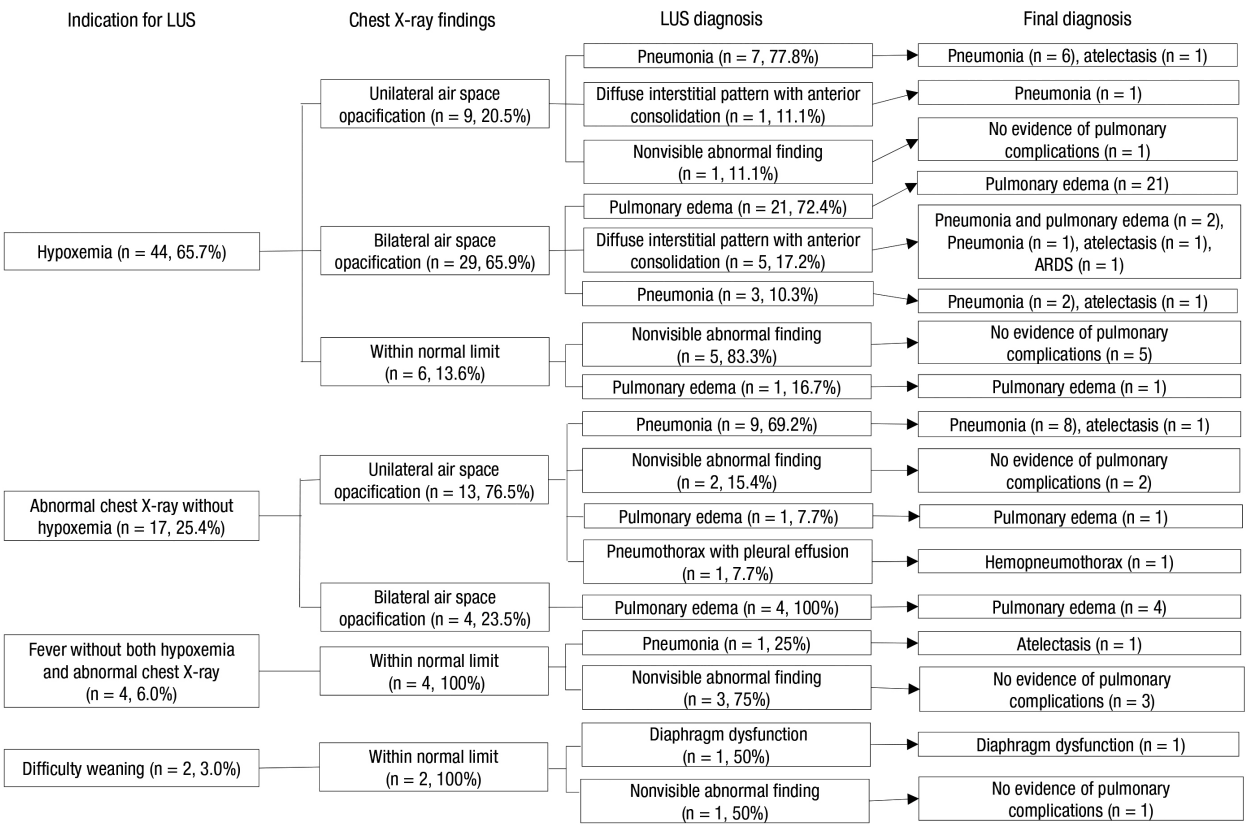
Figure 3.Comparison of a chest radiograph and lung ultrasound image in a representative case. (A) Chest anteroposterior view. Active lesions were not visible. (B) Lung ultrasound of the posterior chest wall. Hypoechoic consolidation with an air bronchogram (arrow) was noted.

Table 1.Definition of diagnosis for LUS in this study
|
Variable |
Right lung
|
Left lung
|
Both lung PLAPS |
|
Upper BLUE point |
Lower BLUE point |
Upper BLUE point |
Lower BLUE point |
|
Pneumonia |
B’ or A/B or C profile |
B’ or A/B or C profile |
B’ or A/B or C profile |
B’ or A/B or C profile |
+ |
|
Pulmonary edema |
B-profile |
B-profile |
B-profile |
B-profile |
± |
|
Diffuse interstitial pattern with anterior consolidation |
B-profile |
B-profile |
B-profile |
B-profile |
± |
|
C profile at least one point of four BLUE points |
|
Table 2.Baseline characteristics of study patients (n = 67)
|
Characteristic |
Value |
|
Age (yr) |
68 (55–78) |
|
Male sex |
47 (70.1) |
|
Body mass index (kg/m2) |
23.9 (21.9–28.4) |
|
No. of examined LUS per person |
1 (1–2) |
|
Comorbidity |
|
|
Coronary artery disease |
14 (20.9) |
|
Atrial fibrillation |
3 (4.5) |
|
Chronic obstructive lung disease |
9 (13.4) |
|
Rib fracture |
7 (10.4) |
|
Previous lung surgery |
2 (3.0) |
|
Chronic kidney disease |
3 (4.5) |
|
Underlying malignancy |
3 (4.5) |
|
Department |
|
|
Neurosurgery |
33 (49.3) |
|
Thoracic surgery |
15 (22.4) |
|
Orthopedic Surgery |
10 (15.0) |
|
Abdominal Surgery |
6 (9.0) |
|
Obstetric surgery |
2 (3.0) |
|
Head and neck surgery |
1 (1.5) |
|
Trauma |
23 (34.3) |
|
Surgical procedure |
49 (73.1) |
Table 3.Findings of LUS in 53 patients with pulmonary edema, pneumonia, and diffuse interstitial pattern with anterior consolidation
|
LUS diagnosis |
Findings of LUS (no. of patients) |
|
Pulmonary edema (n = 27) |
B-profile with bilateral effusion (21) |
|
B-profile without bilateral effusion (6) |
|
Pneumonia (n = 20) |
A/B-profile and C-profile with unilateral effusion (8) |
|
A/B-profile and C-profile without unilateral effusion (8) |
|
A/B-profile with unilateral effusion (3) |
|
C-profile with unilateral effusion (1) |
|
Diffuse interstitial pattern with anterior consolidation (n = 6) |
B-profile and C-profile with bilateral effusion (6) |
Table 4.Final diagnosis according to abnormalities in chest radiography
|
Chest radiographic findings |
Value |
|
Unilateral air space opacification (n = 22) |
|
|
Pneumonia |
15 (68.2) |
|
Atelectasis |
2 (9.1) |
|
Pulmonary edema |
1 (4.5) |
|
Hemopneumothorax |
1 (4.5) |
|
No evidence of pulmonary complications |
3 (13.6) |
|
Bilateral air space opacification (n = 33) |
|
|
Pulmonary edema |
26 (78.8) |
|
Pulmonary edema and pneumonia |
2 (6.1) |
|
Pneumonia |
3 (9.1) |
|
Atelectasis |
1 (3.0) |
|
ARDS |
1 (3.0) |
|
Within normal limit (n = 12) |
|
|
No evidence of pulmonary complications |
9 |
|
Pulmonary edema |
1 |
|
Atelectasis |
1 |
|
Diaphragm dysfunction |
1 |
Table 5.The comparison between final diagnosis and LUS diagnosis
|
Diagnosis for LUS |
Final diagnosis (no. of patients) |
|
Pulmonary edema (n = 27) |
Pulmonary edema (27) |
|
Pneumonia (n = 20) |
Pneumonia (16), atelectasis (4) |
|
Diffuse interstitial pattern with anterior consolidation (n = 6) |
Pneumonia (2), pulmonary edema and pneumonia (2), pulmonary edema (1), ARDS (1) |
|
Pneumothorax with effusion (n = 1) |
Hemopneumothorax (1) |
|
Diaphragm dysfunction (n = 1) |
Diaphragm dysfunction (1) |
|
Nonvisible abnormal findings (n=12) |
No evidence of pulmonary complications (12) |
References
- 1. Sawyer RG, Leon CA. Common complications in the surgical intensive care unit. Crit Care Med 2010;38(9 Suppl):S483-93.ArticlePubMed
- 2. To KB, Napolitano LM. Common complications in the critically ill patient. Surg Clin North Am 2012;92:1519-57.ArticlePubMed
- 3. Hendrikse KA, Gratama JW, ten Hove W, Rommes JH, Schultz MJ, Spronk PE. Low value of routine chest radiographs in a mixed medical-surgical ICU. Chest 2007;132:823-8.ArticlePubMed
- 4. Khan AN, Al-Jahdali H, Al-Ghanem S, Gouda A. Reading chest radiographs in the critically ill (part II): radiography of lung pathologies common in the ICU patient. Ann Thorac Med 2009;4:149-57.ArticlePubMedPMC
- 5. Sperandeo M, Rotondo A, Guglielmi G, Catalano D, Feragalli B, Trovato GM. Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: use and limitations. Radiol Med 2014;119:729-40.ArticlePubMed
- 6. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med 2014;40:57-65.ArticlePubMed
- 7. Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147:1659-70.ArticlePubMed
- 8. Inglis AJ, Nalos M, Sue KH, Hruby J, Campbell DM, Braham RM, et al. Bedside lung ultrasound, mobile radiography and physical examination: a comparative analysis of diagnostic tools in the critically ill. Crit Care Resusc 2016;18:124. ArticlePubMed
- 9. Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med 2011;37:1488-93.ArticlePubMed
- 10. Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A. Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol 2012;78:1282-96.PubMed
- 11. Gardelli G, Feletti F, Nanni A, Mughetti M, Piraccini A, Zompatori M. Chest ultrasonography in the ICU. Respir Care 2012;57:773-81.ArticlePubMed
- 12. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care 2014;4:1. ArticlePubMedPMC
- 13. Chavez MA, Shams N, Ellington LE, Naithani N, Gilman RH, Steinhoff MC, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res 2014;15:50. ArticlePubMedPMC
- 14. Xia Y, Ying Y, Wang S, Li W, Shen H. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis 2016;8:2822-31.ArticlePubMedPMC
- 15. Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033-56.ArticlePubMed
- 16. Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 2011;39:2627-30.ArticlePubMed
- 17. Mongodi S, Via G, Girard M, Rouquette I, Misset B, Braschi A, et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest 2016;149:969-80.ArticlePubMed
- 18. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest 1995;108:1345-8.ArticlePubMed
- 19. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2017;44 Suppl 2:S27-72.Article
- 20. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619-28.ArticlePubMed
- 21. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-111.ArticlePubMedPMCPDF
- 22. Bassin SL, Bleck TP. Barbiturates for the treatment of intracranial hypertension after traumatic brain injury. Crit Care 2008;12:185. ArticlePubMedPMC
- 23. Yagi N, Leblanc M, Sakai K, Wright EJ, Paganini EP. Cooling effect of continuous renal replacement therapy in critically ill patients. Am J Kidney Dis 1998;32:1023-30.ArticlePubMed
- 24. Wang G, Ji X, Xu Y, Xiang X. Lung ultrasound: a promising tool to monitor ventilator-associated pneumonia in critically ill patients. Crit Care 2016;20:320. ArticlePubMedPMCPDF
- 25. Nazerian P, Volpicelli G, Vanni S, Gigli C, Betti L, Bartolucci M, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med 2015;33:620-5.ArticlePubMed
- 26. Hu QJ, Shen YC, Jia LQ, Guo SJ, Long HY, Pang CS, et al. Diagnostic performance of lung ultrasound in the diagnosis of pneumonia: a bivariate meta-analysis. Int J Clin Exp Med 2014;7:115-21.PubMedPMC
- 27. Ye X, Xiao H, Chen B, Zhang S. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS One 2015;10:e0130066.ArticlePubMedPMC
- 28. Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, et al. Lung ultrasound and chest X-ray for detecting pneumonia in an acute geriatric ward. Medicine (Baltimore) 2016;95:e4153. ArticlePubMedPMC
- 29. Ware LB, Matthay MA. Clinical practice: acute pulmonary edema. N Engl J Med 2005;353:2788-96.ArticlePubMed
- 30. Barmparas G, Liou D, Lee D, Fierro N, Bloom M, Ley E, et al. Impact of positive fluid balance on critically ill surgical patients: a prospective observational study. J Crit Care 2014;29:936-41.ArticlePubMed
- 31. Baldi G, Gargani L, Abramo A, D’Errico L, Caramella D, Picano E, et al. Lung water assessment by lung ultrasonography in intensive care: a pilot study. Intensive Care Med 2013;39:74-84.ArticlePubMed
- 32. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004;100:9-15.ArticlePubMed
- 33. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact: an ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997;156:1640-6.ArticlePubMed
- 34. Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014;121:320-7.ArticlePubMed
- 35. Platz E, Lattanzi A, Agbo C, Takeuchi M, Resnic FS, Solomon SD, et al. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail 2012;14:1276-84.ArticlePubMed
- 36. Vignon P, Chastagner C, Berkane V, Chardac E, François B, Normand S, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med 2005;33:1757-63.ArticlePubMed
- 37. Rocco M, Carbone I, Morelli A, Bertoletti L, Rossi S, Vitale M, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand 2008;52:776-84.ArticlePubMed
- 38. Begot E, Grumann A, Duvoid T, Dalmay F, Pichon N, François B, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med 2014;40:1475-80.ArticlePubMed
- 39. Lomas DJ, Padley SG, Flower CD. The sonographic appearances of pleural fluid. Br J Radiol 1993;66:619-24.ArticlePubMed
- 40. Spadaro S, Grasso S, Mauri T, Dalla Corte F, Alvisi V, Ragazzi R, et al. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit Care 2016;20:305. ArticlePubMedPMCPDF
- 41. McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med 2012;366:932-42.ArticlePubMed
- 42. Leeper WR, Leeper TJ, Vogt KN, Charyk-Stewart T, Gray DK, Parry NG. The role of trauma team leaders in missed injuries: does specialty matter? J Trauma Acute Care Surg 2013;75:387-90.ArticlePubMed
Citations
Citations to this article as recorded by

- Lung Ultrasound in the Critically Ill
Jin Sun Cho
The Korean Journal of Critical Care Medicine.2017; 32(4): 356. CrossRef
 , Hyo Jin So, Deok Hee Kim
, Hyo Jin So, Deok Hee Kim , Hyeon-Kyoung Koo
, Hyeon-Kyoung Koo , Hye Kyeong Park
, Hye Kyeong Park , Sung-Soon Lee
, Sung-Soon Lee , Hoon Jung
, Hoon Jung 









 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite