Abstract
-
Background
- Although extracorporeal membrane oxygenation (ECMO) has been used for the treatment of acute high-risk pulmonary embolism (PE), there are limited reports which focus on this approach. Herein, we described our experience with ECMO in patients with acute high-risk PE.
-
Methods
- We retrospectively reviewed medical records of patients diagnosed with acute high-risk PE and treated with ECMO between January 2014 and December 2018.
-
Results
- Among 16 patients included, median age was 51 years (interquartile range [IQR], 38 to 71 years) and six (37.5%) were male. Cardiac arrest was occurred in 12 (75.0%) including two cases of out-of-hospital arrest. All patients underwent veno-arterial ECMO and median ECMO duration was 1.5 days (IQR, 0.0 to 4.5 days). Systemic thrombolysis and surgical embolectomy were performed in seven (43.8%) and nine (56.3%) patients, respectively including three patients (18.8%) received both treatments. Overall 30-day mortality rate was 43.8% (95% confidence interval, 23.1% to 66.8%) and 30-day mortality rates according to the treatment groups were ECMO alone (33.3%, n=3), ECMO with thrombolysis (50.0%, n=4) and ECMO with embolectomy (44.4%, n=9).
-
Conclusions
- Despite the vigorous treatment efforts, patients with acute high-risk PE were related to substantial morbidity and mortality. We report our experience of ECMO as rescue therapy for refractory shock or cardiac arrest in patients with PE.
-
Keywords: embolectomy; extracorporeal membrane oxygenation; pulmonary embolism; shock; thrombolytic therapy; treatment outcome
INTRODUCTION
Acute pulmonary embolism (PE) is the most serious clinical presentation of venous thromboembolism which causes obstructive shock and hemodynamic instability [1-3]. It is stratified based on the early PE-related mortality risk and the high-risk group is defined as the patients who have shock or systemic hypotension [2,3]. Despite the expedited lifesaving treatments such as systemic thrombolysis and surgical embolectomy, unfortunately, acute high-risk PE is associated with significant morbidity and mortality [2-5].
Veno-arterial extracorporeal membrane oxygenation (V-A ECMO) which bypasses both lungs and heart is a potentially lifesaving therapeutic option by providing hemodynamic and respiratory support in patients with acute high-risk PE [5-7]. It may serve as a bridge therapy to systemic thrombolysis or surgical embolectomy or as a stand-alone therapy without primary reperfusion, which supports patients mechanically while waiting for the activation of innate physiologic thrombolysis [5-7]. To date, however, there have been limited reports describing this approach and the literatures still have not provided sufficient evidence whether the ECMO is an effective therapeutic option for the acute high-risk PE [2,3,8]. In this context, we described our experience with ECMO in patients with acute high-risk PE and reviewed the related literatures.
MATERIALS AND METHODS
Study Design and Data Collection
The study was performed at Asan Medical Center, a tertiary referral teaching hospital in Seoul, Korea, where the annual ECMO volume is >200 cases. The data were retrospectively collected from adult patients (≥19 years old) who were diagnosed with acute high-risk PE and treated with ECMO between January 2014 and December 2018. According to the latest American Heart Association and European Society of Cardiology guidelines, the acute high-risk PE was defined as suspected or confirmed acute PE in the presence of shock or persistent arterial hypotension and patients whose systolic blood pressure <90 mmHg or drop by ≥40 mmHg for >15 minutes were regarded as having a persistent arterial hypotension [2,3]. In our institution, ECMO was performed by a specialized ECMO team which consists of cardiothoracic surgeons and perfusionists. Because we included patients with acute high-risk PE who require urgent cardiopulmonary and respiratory support, peripherally inserted V-A ECMO of which drainage cannulation at femoral vein and return cannulation at femoral artery was primarily considered as an initial configuration in all cases.
Data on baseline characteristics were retrieved at the time of diagnosis, which include age, gender, body mass index, smoking history, comorbidities, predisposing factors for venous thromboembolism, presenting symptoms and signs, and diagnostic modalities. We also investigated major procedures performed for the treatment of acute high-risk PE including systemic thrombolysis and surgical embolectomy. Clinical outcomes were compared in terms of 30-day mortality rate and ECMO weaning rate according to the treatment modalities; thrombolysis group vs. nonthrombolysis group and embolectomy group vs. non-embolectomy group. Complications were investigated and compared according to the treatment groups as well.
The study was performed according to the Helsinki Declaration and approved by the Institutional Review Board of Asan Medical Center (IRB No. 2019-0359). The informed consent was waived because of the retrospective nature of the study.
Statistical Analysis
Continuous variables were summarized using means with standard deviations and categorical variables were summarized using frequencies with proportions. Kaplan-Meier curves were plotted for the survival analyses. Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline Characteristics
We identified 16 patients with acute high-risk PE who were treated with ECMO between January 2014 and December 2018. Table 1 describes the baseline characteristics of included patients. The median age was 51 years (interquartile range [IQR], 38 to 71 years) and six patients (37.5%) were male. The most common predisposing factor for venous thromboembolism was recent hospitalization (n=7, 43.7%) followed by active cancer (n=6, 37.5%) and recent invasive vascular procedures (n=5, 31.2%). Four patients (25.0%) were pregnant at the time of diagnosis and previous history of venous thromboembolism was reported in one patient (6.2%).
Initial Presentation and Diagnosis
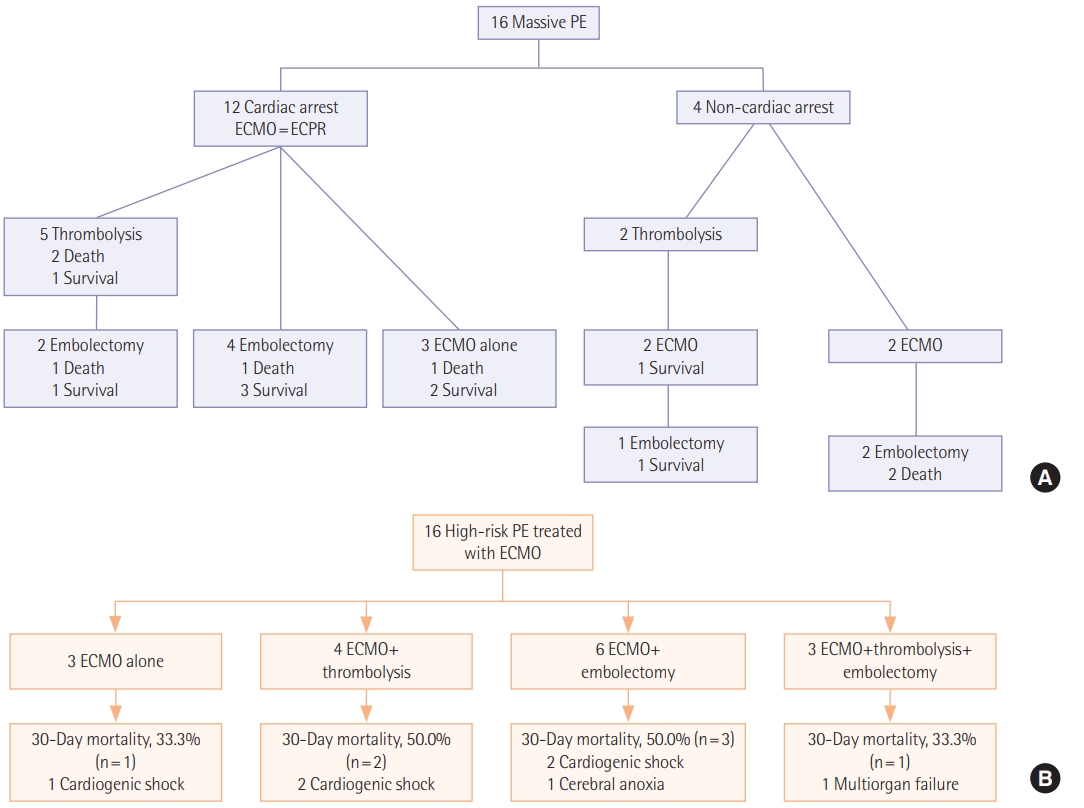
Initial symptoms and signs and the diagnostic modalities are described in Table 2. The most common initial presentation was dyspnea (n=6, 37.5%) followed by hemodynamic collapse (n=4, 25.0%). Cardiac arrest was reported in 12 (75.0%) including two cases occurred at out-of-hospital in Figure 1. Among 13 patients who performed computed tomography (CT) angiography, right ventricle strain pattern which was defined as the ratio of right ventricle and left ventricle ratio >1 was observed in 12 patients (92.3%). The diagnosis was confirmed without CT angiography in three patients (18.8%). Two patients were suspected of acute PE on echocardiography and the diagnosis was confirmed during surgical embolectomy. A patient was presented with cardiac arrest and initially suspected of acute coronary syndrome. He was transferred for coronary angiography, but the there was no abnormality in coronary arteries and acute PE was diagnosed during pulmonary angiography. Echocardiography was performed in all patients of which 15 (93.8%) showed right ventricular dilatation and dysfunction.
Therapeutic Procedures
Procedures performed for the treatment of acute high-risk PE are described in Table 3. All patients required mechanical support with V-A ECMO and the median ECMO duration was 1.5 days (IQR, 0.0 to 4.5 days). Among 16 patients included, three (18.8%) were treated with ECMO alone and 13 (81.3%) were bridged to one or more primary reperfusion therapies such as systemic thrombolysis and surgical embolectomy. Systemic thrombolysis and surgical embolectomy were performed in seven patients (43.8%) and nine patients (56.3%), respectively including three patients (18.8%) who received both treatments; in these patients, systemic thrombolysis was followed by surgical embolectomy because of persistent shock and systemic hypotension. No catheter-based thrombolysis was performed because it was not available in our hospital.
Clinical Outcomes and Complications
The overall 30-day mortality was occurred in seven patients (43.8%) and the ECMO was successfully weaned in 10 patients (62.5%) (Table 4, Figure 2). The most common complication was bleeding (n=9, 56.3%) which requires transfusion of ≥2 packed red blood cells. Cerebrovascular accident and arterial ischemia were reported in one patient (6.2%), respectively, and both were assessed to be associated with ECMO support.
In Figure 1, we described 30-day mortalities and causes of death according to the treatment strategies. Despite the vigorous treatment efforts, substantial mortalities were reported in each treatment group (33.3%–50.0%). The most common cause of death was cardiogenic shock (n=5, 71.4%). Cerebral anoxia and multiorgan failure were reported in a case (6.3%), respectively. We also compared clinical outcomes and complications according to the treatment groups; 30-day mortality rate was 43.8% (95% confidence interval, 23.1% to 66.8%) and 30-day mortality rates according to the treatment groups was ECMO alone (n=3, 33.3%), ECMO with thrombolysis (n=4, 50.0%) and ECMO with embolectomy (n=9, 44.4%) (Table 4).
We showed outcome of patients by initial cardiac arrest or not (Table 5). Besides four peripartum patients were included. After C-sec, PE developed three patients and a patient after D&C due to ectopic pregnancy. We did all four patients ECPR, one patient died during extracorporeal cardiopulmonary resuscitation, and three patients survived. Among three patients, one patient received thrombolysis, one for embolectomy, and one for ECMO alone.
DISCUSSION
The management of acute high-risk PE requires multifaceted approaches including hemodynamic and respiratory support in addition to anticoagulation and primary reperfusion therapy [2,3]. Several approaches have been suggested to provide hemodynamic and respiratory support, which includes fluid challenge, use of vasopressors and inotropes, inhalation of nitric oxide, and mechanical ventilation with minimal positive end expiratory pressure. However, none has been proved to provide sufficient hemodynamic and respiratory support in patients with acute high-risk PE [2,3,9-12]. Recently, there is an increasing attention to the utilization of ECMO in the treatment of acute PE which causes hemodynamic instability [5]. But the notion is only supported by occasional case reports and patient series and the literatures still have not provided sufficient evidence whether the ECMO is an effective option for the treatment of acute high-risk PE [2,3,5-8,13-15].
In this consecutive case series, we presented our data on 16 patients with confirmed acute high-risk PE who required both hemodynamic and respiratory support with V-A ECMO. Systemic thrombolysis and surgical embolectomy were performed in seven and nine patients, respectively including three patients who received both treatments. Despite the vigorous therapeutic efforts, patients with acute high-risk PE had significant morbidity and mortality and seven patients (43.8%) died within 30 days from the initial diagnosis. The overall 30-day mortality rate, 43.8%, reported in our study seems to be high, but is similar to the results reported in other studies which support the potential benefit of ECMO in patients with acute high-risk PE [6,7,13-15]. Moreover, in the present study, three-quarters of patients were initially presented with cardiac arrest which is a well-known risk factor for mortality even when full standard resuscitative efforts are employed [5,16]. Considering the higher mortality rate in patients with acute PE who developed cardiac arrest, which ranges from 52% to 65% [17], further study is needed for the potential benefit of ECMO in patients with acute high-risk PE.
Historically surgical embolectomy was the treatment of choice for acute PE which causes shock or systemic hypotension, but less invasive procedures such as systemic thrombolysis have been introduced during last decades [5,18,19]. Currently, both treatments are recommended by the latest clinical guidelines and, in a recent systematic review, there is no evidence that one is superior to the other treatment particularly in patients treated with ECMO [2,3,5]. In the present study, 30-day mortality rates and ECMO weaning rates were similar among treatments.
Treatment with ECMO alone also may be considered in some patients with acute high-risk PE. It has been shown that the recovery of right ventricle and hemodynamic improvement may be achieved within 5 days from the ECMO support by the activation of innate physiologic thrombolysis [17]. In our study, three patients were treated with ECMO alone without any primary reperfusion therapy. Although one of them, who was initially presented with out-of-hospital cardiac arrest and required >30 minutes until the initiation of ECMO support, died within a few hours after admission, the remaining are survived without other invasive procedures. This approach may be beneficial by avoiding potential risks of primary reperfusion therapy such as major bleeding and surgical site infection, but currently there are limited data regarding who will be successfully bridged to recovery or who will require primary reperfusion therapy. Further studies are required to assess the clinical outcomes and prognostic factors in patients with acute high-risk PE, treated with ECMO support alone.
There are several limitations in our study. The study was retrospectively performed in a single center and may be prone to bias. The relatively small number of patients included in the study also limit the generalization of results. Nonetheless, it should be noted that this is an area where the randomized controlled trials are virtually impossible and the results from cases reports and patient series are important to guide clinical practices.
In conclusion, despite the vigorous treatment efforts, patients with acute high-risk PE were related to substantial morbidity and mortality. We report our experience of ECMO as rescue therapy for refractory shock or cardiac arrest in patients with PE. Further investigations are required in larger samples to confirm our findings.
KEY MESSAGES
▪Overall 30-day mortality rate was 43.8% in patients with acute high-risk pulmonary embolism who required hemodynamic and respiratory support with extracorporeal membrane oxygenation (ECMO).
▪We divided three group of patients such as ECMO alone, ECMO with thrombolysis, and ECMO with embolectomy.
▪ECMO was treated in conjunction with either systemic thrombolysis or surgical embolectomy in patients with acute high-risk pulmonary embolism.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conceptualization: SBH. Data curation: YNO, DKO, JSL, SHJ, PJK. Formal analysis: YNO, DKO, SBH. Methodology: YNO, DKO, YK, CML, JWH, JSL, SBH. Project administration: YNO, DKO, SBH. Visualization: YNO, DKO. Writing - original draft: YNO, DKO. Writing - review & editing: all authors.
Figure 1.(A) A timeline of treatment strategies and 30-day mortalities. (B) A flowchart of treatment strategies and 30-day mortalities with causes of death. PE: pulmonary embolism; ECMO: extracorporeal membrane oxygenation; ECPR: extracorporeal cardiopulmonary resuscitation.
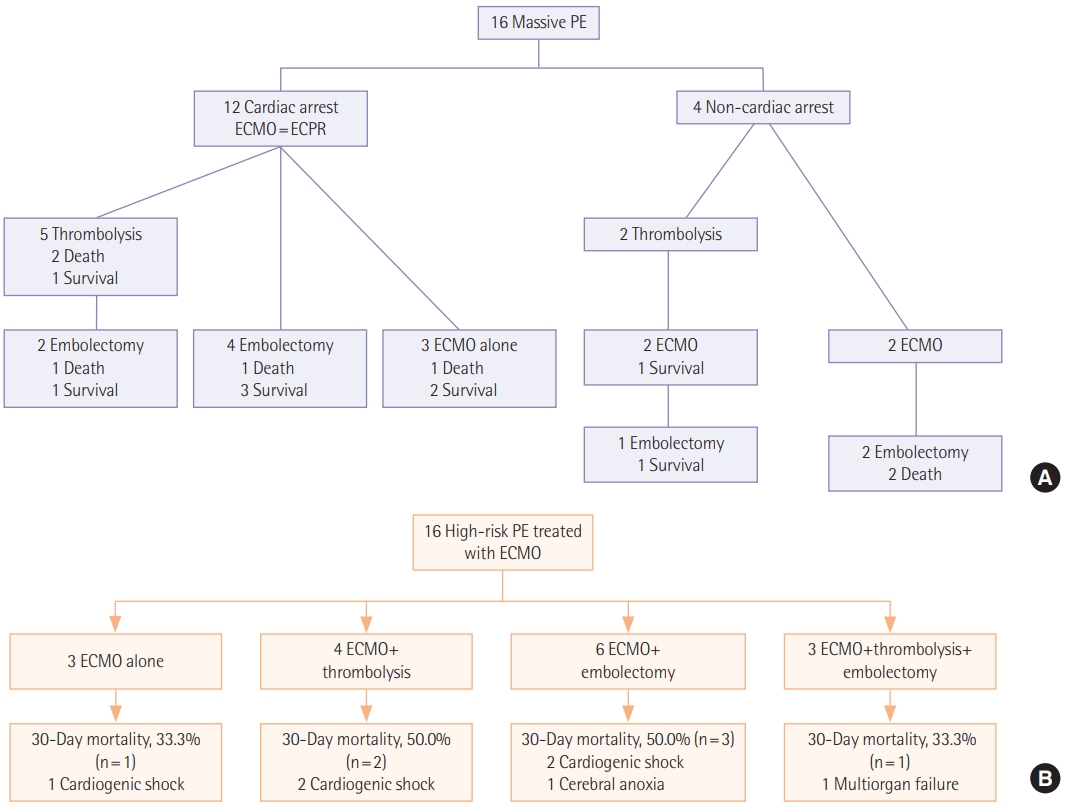
Figure 2.Thirty-day mortality. CI: confidence interval.

Table 1.Baseline characteristics of the included patients
|
Variable |
Value (n=16) |
|
Age (yr) |
51 (38–71) |
|
Male |
6 (37.5) |
|
Body mass index (kg/m2) |
25.0 (22.6–32.7) |
|
Smoking |
2 (12.5) |
|
Comorbidity |
|
|
Diabetes |
4 (25.0) |
|
Hypertension |
4 (25.0) |
|
Chronic kidney disease |
0 |
|
Predisposing factor |
|
|
Recent hospitalization |
7 (43.7) |
|
Active cancer |
6 (37.5) |
|
Recent invasive procedure |
5 (31.2) |
|
Pregnancy |
4 (25.0) |
|
Immobilization |
3 (18.8) |
|
History of venous thromboembolism |
1 (6.2) |
|
Recent trauma |
1 (6.2) |
|
Hormone therapy |
0 |
|
Thrombophilia |
0 |
Table 2.Initial presentation and diagnosis of acute high-risk pulmonary embolism
|
Variable |
Value (n=16) |
|
Initial symptom |
|
|
Dyspnea |
6 (37.5) |
|
Hemodynamic collapse |
4 (25.0) |
|
Syncope |
3 (18.8) |
|
Altered mentality |
2 (12.5) |
|
Leg pain or swelling |
1 (6.2) |
|
Cardiac arrest |
12 (75.0) |
|
In-hospital |
10 (83.3) |
|
Out-of-hospital |
2 (16.7) |
|
CT angiography |
13 (81.2) |
|
Saddle pulmonary embolism on CT |
9 (69.2) |
|
RV strain on CT |
12 (92.3) |
|
Echocardiography |
16 (100.0) |
|
RV dysfunction |
15 (93.8) |
|
RV dilatation |
15 (93.8) |
|
LV ejection fraction (%) |
21 (9–53) |
|
Laboratoryfinding |
|
|
D-dimer (μg/ml) |
24.1 (6.7–35.2) |
|
CK-MB (ng/ml) |
4.0 (1.8–9.2) |
|
Troponin I (pg/ml) |
0.3 (0.1–1.2) |
|
BNP (pg/ml) |
171 (65–347) |
|
Lactate (mmol/L) |
9.4 (4.9–13.3) |
Table 3.Treatment of acute high-risk pulmonary embolism
|
Variable |
Value (n=6) |
|
ECMO |
16 (100.0) |
|
Duration (day) |
1.5 (0.0–4.5) |
|
V-A ECMO |
16 (100.0) |
|
Central V-A ECMO |
2 (12.5) |
|
IVC filter |
2 (12.5) |
|
Treatment strategy |
|
|
ECMO alone |
3 (18.8) |
|
ECMO+systemic thrombolysis |
4 (25.0) |
|
ECMO+surgical embolectomy |
6 (37.5) |
|
ECMO+systemic thrombolysis+surgical embolectomy |
3 (18.8) |
Table 4.Clinical outcomes and complications according to treatment strategies
|
Variable |
ECMO alone (n=3) |
ECMO with thrombolysis (n=4) |
ECMO with embolectomy (n=9) |
|
30-Day mortality |
1 (33.3) |
2 (50.0) |
4 (44.4) |
|
ECMO weaning |
2 (66.7) |
2 (50.0) |
6 (66.7) |
|
Bleeding |
1 (33.3) |
1 (25.0) |
7 (77.8) |
|
Cerebrovascular event |
0 |
0 |
1 (11.1) |
|
Wound infection |
0 |
1 (25.0) |
1 (11.1) |
|
Arterial ischemia |
0 |
0 |
1 (11.1) |
|
Recurrent DVT |
0 |
0 |
1 (11.1) |
|
CTEPH |
0 |
0 |
0 |
Table 5.ECMO outcomes and ICU events according to CPR
|
Outcome/event |
Non-CPR (n=4) |
CPR (n=12) |
|
30-Day mortality |
2 (50.0) |
5 (41.7) |
|
In-hospital mortality |
2 (50.0) |
7 (58.3) |
|
Total mortality |
2 (50.0) |
7 (58.3) |
|
ECMO duration (day) |
5.2±5.3 |
3.0±4.6 |
|
ICU LOS (day) |
8.5±5.3 |
12.3±15.2 |
|
Hospital LOS (day) |
25.8±19.9 |
46.7±56.8 |
|
Thrombolysis |
2 (50.0) |
5 (41.7) |
|
Embolectomy |
3 (75.0) |
6 (50.0) |
|
Acute kidney injury requiring RRT |
2 (50.0) |
7 (58.3) |
|
Cerebrovascular event |
0 |
1 (8.3) |
|
Wound infection |
0 |
2 (16.7) |
|
Artery ischemia |
0 |
1 (8.3) |
|
Recurrent DVT |
1 (25.0) |
0 |
|
CTEPH |
0 |
0 |
References
- 1. Aissaoui N, Konstantinides S, Meyer G. What’s new in severe pulmonary embolism? Intensive Care Med 2019;45:75-7.ArticlePubMedPDF
- 2. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69.ArticlePubMedPDF
- 3. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830.ArticlePubMedPMC
- 4. Logan JK, Pantle H, Huiras P, Bessman E, Bright L. Evidencebased diagnosis and thrombolytic treatment of cardiac arrest or periarrest due to suspected pulmonary embolism. Am J Emerg Med 2014;32:789-96.ArticlePubMedPMC
- 5. Yusuff HO, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion 2015;30:611-6.ArticlePubMedPMC
- 6. Corsi F, Lebreton G, Bréchot N, Hekimian G, Nieszkowska A, Trouillet JL, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care 2017;21:76. ArticlePubMedPMCPDF
- 7. Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide-Chevronnay L, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J 2018;39:4196-204.ArticlePubMedPDF
- 8. Galiè N, Palazzini M, Manes A. Extracorporeal cardiopulmonary support in acute high-risk pulmonary embolism: still waiting for solid evidence. Eur Heart J 2018;39:4205-7.ArticlePubMedPDF
- 9. Manier G, Castaing Y. Influence of cardiac output on oxygen exchange in acute pulmonary embolism. Am Rev Respir Dis 1992;145:130-6.ArticlePubMedPMC
- 10. Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med 1999;27:540-4.ArticlePubMedPMC
- 11. Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F. Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med 1997;23:1089-92.ArticlePubMedPMCPDF
- 12. Szold O, Khoury W, Biderman P, Klausner JM, Halpern P, Weinbroum AA. Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: a report of four patients and review of the literature. Lung 2006;184:1-5.ArticlePubMedPDF
- 13. Dolmatova EV, Moazzami K, Cocke TP, Elmann E, Vaidya P, Ng AF, et al. Extracorporeal membrane oxygenation in massive pulmonary embolism. Heart Lung 2017;46:106-9.ArticlePubMed
- 14. Kjaergaard B, Kristensen JH, Sindby JE, de Neergaard S, Rasmussen BS. Extracorporeal membrane oxygenation in lifethreatening massive pulmonary embolism. Perfusion 2019;267659119830014.ArticlePDF
- 15. George B, Parazino M, Omar HR, Davis G, Guglin M, Gurley J, et al. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation 2018;122:1-5.ArticlePubMed
- 16. Sakuma M, Nakamura M, Yamada N, Nakano T, Shirato K. Percutaneous cardiopulmonary support for the treatment of acute pulmonary embolism: summarized review of the literature in Japan including our own experience. Ann Vasc Dis 2009;2:7-16.ArticlePubMedPMC
- 17. Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma 2007;62:570-6.ArticlePubMed
- 18. Leacche M, Unic D, Goldhaber SZ, Rawn JD, Aranki SF, Couper GS, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005;129:1018-23.ArticlePubMedPMC
- 19. Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004;110:744-9.ArticlePubMed
Citations
Citations to this article as recorded by

- Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest: an overview of current practice and evidence
Samir Ali, Christiaan L. Meuwese, Xavier J. R. Moors, Dirk W. Donker, Anina F. van de Koolwijk, Marcel C. G. van de Poll, Diederik Gommers, Dinis Dos Reis Miranda
Netherlands Heart Journal.2024; 32(4): 148. CrossRef - Integration of Extracorporeal Membrane Oxygenation into the Management of High-Risk Pulmonary Embolism: An Overview of Current Evidence
Romain Chopard, Raquel Morillo, Nicolas Meneveau, David Jiménez
Hämostaseologie.2024;[Epub] CrossRef - Evidence-Based Management of Massive and Submassive Pulmonary Embolism
Sara Al-Juboori, Tareq Alzaher, Hashem Al Omari, Sufyan Al Gammaz, Mazen Al-Qadi
JAP Academy Journal.2024;[Epub] CrossRef - Mechanical Support in High-Risk Pulmonary Embolism: Review Article
Amer N. Kadri, Razan Alrawashdeh, Mohamad K. Soufi, Adam J. Elder, Zachary Elder, Tamam Mohamad, Eric Gnall, Mahir Elder
Journal of Clinical Medicine.2024; 13(9): 2468. CrossRef - Extracorporeal membrane oxygenation for large pulmonary emboli
Timothy J. George, Jenelle Sheasby, Rahul Sawhney, J. Michael DiMaio, Aasim Afzal, Dennis Gable, Sameh Sayfo
Baylor University Medical Center Proceedings.2023; 36(3): 314. CrossRef - Surgical Management and Mechanical Circulatory Support in High-Risk Pulmonary Embolisms: Historical Context, Current Status, and Future Directions: A Scientific Statement From the American Heart Association
Joshua B. Goldberg, Jay Giri, Taisei Kobayashi, Marc Ruel, Alexander J.C. Mittnacht, Belinda Rivera-Lebron, Abe DeAnda, John M. Moriarty, Thomas E. MacGillivray
Circulation.2023;[Epub] CrossRef - Life-threatening pulmonary embolism: overview and management
Nizar Osmani, Jonathan Marinaro, Sundeep Guliani
International Anesthesiology Clinics.2023; 61(4): 35. CrossRef - Extracorporeal Membrane Oxygenation for Pulmonary Embolism: A Systematic Review and Meta-Analysis
Jonathan Jia En Boey, Ujwal Dhundi, Ryan Ruiyang Ling, John Keong Chiew, Nicole Chui-Jiet Fong, Ying Chen, Lukas Hobohm, Priya Nair, Roberto Lorusso, Graeme MacLaren, Kollengode Ramanathan
Journal of Clinical Medicine.2023; 13(1): 64. CrossRef - Pulmonary ECMO-ism: Let’s add PEA to ECPR indications
Zachary Shinar, Alice Hutin
Resuscitation.2022; 170: 293. CrossRef - Combined use of extracorporeal membrane oxygenation with interventional surgery for acute pancreatitis with pulmonary embolism: A case report
Ling-Ling Yan, Xiu-Xiu Jin, Xiao-Dan Yan, Jin-Bang Peng, Zhuo-Ya Li, Bi-Li He
World Journal of Clinical Cases.2022; 10(12): 3899. CrossRef - Pulmonary Embolism Complicated With Cardiopulmonary Arrest Treated With Combination of Thrombolytics and Aspiration Thrombectomy
Taylor C. Remillard, Zain Kassam, Maks Coven, Aditya Mangla, Zoran Lasic
JACC: Case Reports.2022; 4(10): 576. CrossRef - Anesthetic management for intraoperative acute pulmonary embolism during inferior vena cava tumor thrombus surgery: A case report
Pei-Yu Hsu, En-Bo Wu
World Journal of Clinical Cases.2022; 10(15): 5111. CrossRef - Percutaneous mechanical thrombectomy and extracorporeal membranous oxygenation: A case series
Haytham Mously, Jamal Hajjari, Tarek Chami, Tarek Hammad, Robert Schilz, Teresa Carman, Yakov Elgudin, Yasir Abu‐Omar, Marc P. Pelletier, Mehdi H. Shishehbor, Jun Li
Catheterization and Cardiovascular Interventions.2022; 100(2): 274. CrossRef - Clinical Experiences of High-Risk Pulmonary Thromboembolism Receiving Extracorporeal Membrane Oxygenation in Single Institution
Joonyong Jang, So-My Koo, Ki-Up Kim, Yang-Ki Kim, Soo-taek Uh, Gae-Eil Jang, Wonho Chang, Bo Young Lee
Tuberculosis and Respiratory Diseases.2022; 85(3): 249. CrossRef - Management of High-Risk Pulmonary Embolism: What Is the Place of Extracorporeal Membrane Oxygenation?
Benjamin Assouline, Marie Assouline-Reinmann, Raphaël Giraud, David Levy, Ouriel Saura, Karim Bendjelid, Alain Combes, Matthieu Schmidt
Journal of Clinical Medicine.2022; 11(16): 4734. CrossRef - Optimal reperfusion strategy in acute high-risk pulmonary embolism requiring extracorporeal membrane oxygenation support: a systematic review and meta-analysis
Romain Chopard, Peter Nielsen, Fabio Ius, Serghei Cebotari, Fiona Ecarnot, Hugo Pilichowski, Matthieu Schmidt, Benedict Kjaergaard, Iago Sousa-Casasnovas, Mehrdad Ghoreishi, Rajeev L. Narayan, Su Nam Lee, Gregory Piazza, Nicolas Meneveau
European Respiratory Journal.2022; 60(5): 2102977. CrossRef - Use of extracorporeal membrane oxygenation in high‐risk acute pulmonary embolism: A systematic review and meta‐analysis
Luca Baldetti, Alessandro Beneduce, Lorenzo Cianfanelli, Giulio Falasconi, Luigi Pannone, Francesco Moroni, Angela Venuti, Stefania Sacchi, Mario Gramegna, Vittorio Pazzanese, Francesco Calvo, Guglielmo Gallone, Matteo Pagnesi, Alberto Maria Cappelletti
Artificial Organs.2021; 45(6): 569. CrossRef - Institutional Experience With Venoarterial Extracorporeal Membrane Oxygenation for Massive Pulmonary Embolism: A Retrospective Case Series
Maxwell A. Hockstein, Christina Creel-Bulos, Joshua Appelstein, Craig S. Jabaley, Michael J. Stentz
Journal of Cardiothoracic and Vascular Anesthesia.2021; 35(9): 2681. CrossRef - Venoarterial Extracorporeal Membrane Oxygenation in Massive Pulmonary Embolism-Related Cardiac Arrest: A Systematic Review*
John Harwood Scott, Matthew Gordon, Robert Vender, Samantha Pettigrew, Parag Desai, Nathaniel Marchetti, Albert James Mamary, Joseph Panaro, Gary Cohen, Riyaz Bashir, Vladimir Lakhter, Stephanie Roth, Huaqing Zhao, Yoshiya Toyoda, Gerard Criner, Lisa Moor
Critical Care Medicine.2021; 49(5): 760. CrossRef - Adult Langerhans histiocytosis with rare BRAF mutation complicated by massive pulmonary embolism
Salma Hassan, Christina Fanola, Amy Beckman, Faqian Li, Andrew C. Nelson, Michael Linden, Joan D. Beckman
Thrombosis Research.2020; 193: 207. CrossRef - Efficacy and safety of extracorporeal membrane oxygenation for high-risk pulmonary embolism: A systematic review and meta-analysis
Matteo Pozzi, Augustin Metge, Anthony Martelin, Caroline Giroudon, Justine Lanier Demma, Catherine Koffel, William Fornier, Pascal Chiari, Jean Luc Fellahi, Jean Francois Obadia, Xavier Armoiry
Vascular Medicine.2020; 25(5): 460. CrossRef - Evidence-Based Minireview: Advanced therapies and extracorporeal membrane oxygenation for the management of high-risk pulmonary embolism
Radhika Gangaraju, Frederikus A. Klok
Hematology.2020; 2020(1): 195. CrossRef
 , Dong Kyu Oh1
, Dong Kyu Oh1 , Younsuck Koh1
, Younsuck Koh1 , Chae-Man Lim1
, Chae-Man Lim1 , Jin-Won Huh1
, Jin-Won Huh1 , Jae Seung Lee1
, Jae Seung Lee1 , Sung-Ho Jung2
, Sung-Ho Jung2 , Pil-Je Kang2
, Pil-Je Kang2 , Sang-Bum Hong1
, Sang-Bum Hong1





 KSCCM
KSCCM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite