Abstract
-
Background
- Although the use of volatile sedatives in the intensive care unit (ICU) is increasing in Europe, it remains infrequent in Asia. Therefore, there are no clinical guidelines available. This study investigates the proper initial concentration of sevoflurane, a volatile sedative that induces a Richmond agitation-sedation scale (RASS) score of –2 to –3, in patients who underwent head and neck surgery with tracheostomy. We also compared the amount of postoperative opioid consumption between volatile and intravenous (IV) sedation.
-
Methods
- We planned a prospective study to determine the proper initial sevoflurane concentration and a retrospective analysis to compare postoperative opioid consumption between volatile sedation and propofol sedation. Patients scheduled for head and neck surgery with tracheostomy and subsequent postoperative sedation in the ICU were enrolled.
-
Results
- In this prospective study, the effective dose 50 (ED50) of initial end-tidal sevoflurane concentration was 0.36% (95% confidence interval [CI], 0.20 to 0.60%), while the ED 95 was 0.69% (95% CI, 0.60 to 0.75%) based on isotonic regression methods. In this retrospective study, remifentanil consumption during postoperative sedation was significantly lower in the sevoflurane group (2.52±1.00 µg/kg/hr, P=0.001) than it was in the IV propofol group (3.66±1.30 µg/kg/hr).
-
Conclusions
- We determined the proper initial end-tidal concentration setting of sevoflurane for patients with tracheostomy who underwent head and neck surgery. Postoperative sedation with sevoflurane appears to be a valid and safe alternative to IV sedation with propofol.
-
Keywords: AnaConDa; analgesics; anesthesia; postoperative period; sevoflurane
INTRODUCTION
Sedation is a frequent procedure in the intensive care unit (ICU). Providing proper sedation to critically ill patients is very important, because it not only reduces their anxiety and discomfort but also affects their outcomes and survival rates. The current guidelines recommend light sedation and daily sedative interruption protocols [1]. In patients who have undergone head and neck flap surgery, it is essential to maintain airway patency to maintain the viability of the implanted tissue flap. These patients are usually maintained on mechanical ventilation for days [2,3]. Moderate sedation to achieve a Richmond agitation-sedation scale (RASS) score of –2 to –3 is typically used to minimize patient motion and ensure patient comfort. Immediate neurologic examinations after sedative interruption are also important to detect neurologic abnormalities, such as stroke [4,5]. The ideal sedatives can easily achieve the desired level of sedation, and rapid wakening when the infusion is stopped.
Non-benzodiazepine sedatives, such as propofol or dexmedetomidine, can improve patient outcomes in the ICU and reduce ICU lengths of stay, the duration of mechanical ventilation, and the incidence of delirium. These sedatives can also improve long-term outcomes, such as 90-day mortality, cognitive and physical functioning, institutionalization, and psychologic dysfunction [1]. In addition, long-term high-dose benzodiazepine infusion for sedation can lead to prolonged sedation after the infusion is discontinued [6]. However, sufficient moderate sedation is difficult to obtain with dexmedetomidine alone. For that reason, propofol infusion has been used for postoperative sedation and rapid recovery in patients undergoing head and neck surgery. However, propofol can cause serious hemodynamic instability, including hypotension. Furthermore, long-term high-dose propofol infusion can induce propofol infusion syndrome, which can lead to metabolic acidosis, cardiac and skeletal muscle injury, kidney injury, and even death [7-9].
Several recent studies have addressed postoperative volatile sedation [10-14]. Volatile agents have been widely used in the operating room for general anesthesia. However, their use requires a ventilator with a classic vaporizer or a closed anesthesia system. Applying a volatile agent to an open non-rebreathing ICU ventilator is not cost-effective and can cause environmental pollution. Therefore, inhalation sedation is mainly used in the operating room and not in the ICU. An anesthetic conserving device (ACD; AnaConDa, Sedana Medical, Danderyd, Sweden) is a simple disposable device that allows inhaled anesthetics to be vaporized and delivered using any non-rebreathing mechanical ventilator. Liquid form sevoflurane is infused using a 50-mL syringe and vaporized by an evaporator rod. The gas from sevoflurane is mixed with the gas from the inspiratory limb, and delivered to the patient’s lungs during inspiration. During the expiratory phase, 90% of the exhaled volatile agent is adsorbed and reused in the next inspiration [15]. The ACD enabled the use of inhalation sedatives without an anesthetic machine, making it possible to use volatile sedation in the ICU.
Although the use of volatile sedatives in the ICU is increasing in Europe, it is still rare in Asia. There are no clinical capacity guidelines regarding the use of volatile sedatives in the ICU in Asia. Therefore, in this study, we investigated the proper initial concentration of sevoflurane in patients who underwent head and neck surgery with tracheostomy. Sevoflurane is a volatile sedative that induces a RASS score of –2 to –3. We also compared the amount of postoperative opioid consumption between patients receiving volatile and intravenous (IV) sedation.
MATERIALS AND METHODS
Ethical Considerations
This study was approved by Institutional Review Board of Severance Hospital (IRB No. 4-2018-0065). Written informed consent was obtained from all patients. We registered this study as a clinical trial (Clinicaltrials.gov identifier NCT-03559920).
Study Design
We planned both a prospective study to determine the proper initial sevoflurane concentration, and a retrospective analysis to compare volatile sedation with IV sedation.
Prospective Study
Patients
Patients who were scheduled for elective head and neck surgery with tracheostomy followed by sedation and postoperative mechanical ventilation in the surgical ICU at our hospital from April 2018 to October 2018 were enrolled. The inclusion criteria included patients over 19 years with American Society of Anesthesiologists physical status I–III. The exclusion criteria were as follows: family history of malignant hyperthermia, chronic kidney disease (estimated glomerular filtration rate < 30 or patients on dialysis), moderate to severe liver disease (aspartate transaminase and alanine transaminase levels > 200 IU/L) or a diagnosis of chronic liver disease [16], pregnancy, and refusal to participate.
Sedation procedure
First, we conducted a prospective study. During surgery, anesthesia in patients was maintained using an inhaled anesthetic agent and remifentanil. After surgery, patients were injected with 0.05 mg/kg of midazolam to keep them sedated while they were transferred from the operating room to the ICU, which took 10–15 minutes. Upon arrival to the ICU, the standard monitoring devices were attached. During mechanical ventilation, the tidal volume was set at 6–7 mL per ideal body weight (kg). The respiratory rate was adjusted so that the end-tidal carbon dioxide (CO2) could be maintained at 35–45 mm Hg. The fraction of inspired oxygen was initially set at 0.4 and the positive end-expiratory pressure was set at 5 mm Hg. These parameters were adjusted as needed for proper oxygenation. Hypotension was defined as a mean arterial blood pressure <65 mm Hg or 20% less than the baseline values [17]. When hypotension lasted for more than 1 minute, additional fluid or norepinephrine was administered to keep the mean arterial blood pressure > 65 mm Hg or to avoid > 20% decrease in the baseline blood pressure of the patient.
Subsequently, the ACD (AnaConDa) was applied to deliver the sevoflurane. In order to prime the injecting adapter, a bolus of 1.5 ml sevoflurane was initially administered. The end-tidal CO2 and sevoflurane concentration were tracked on the gas monitor, and the correct operation of the ACD (AnaConDa) was monitored. For analgesia during sedation, 0.1–0.2 µg/kg/min of remifentanil was infused to maintain patients at a Critical-Care Pain Observation Tool (CPOT) score of < 3.
Outcome measurements
The target depth of sedation was adjusted to a RASS score of –2 to –3. Patients responded to voice commands or opened their eyes, but eye contact was maintained for 10 s or less [18]. According to previous research results, the first patient’s end-tidal sevoflurane concentration target was set at 0.5% according to the manufacturer’s recommendations [19,20]. The infusion rate was adjusted at 0.5–5 ml/hr to reach a measured end-tidal sevoflurane concentration of 0.5% 30 minutes after the initial sedation. We estimated the patients’ sedation level at 30 minutes. Sedation was considered successful when the patient reached a RASS score of –2 to –3. In order to determine the minimum effective drug concentration, the next patient’s end-tidal sevoflurane concentration target was set at 0.4%, which was 0.1% less than that of the previous patient. If the sedation level at 30 minutes was too deep (below RASS –4), the next patient’s target concentration was set at 0.3%, which was 0.1% less than that of the previous patient. Conversely, if the sedation level was too shallow (RASS –1 or more agitated) when the target end-tidal sevoflurane concentration was reached, the target concentration was set at 0.6%, which was 0.1% greater than that of the previous patient. By repeating these procedures, we obtained the appropriate concentration of end-tidal sevoflurane (ED 50 and ED 95) at the initial phase of sedation that can induce a RASS score of –2 to –3. The ED 50 was defined as the effective initial end-tidal sevoflurane concentration for achieving the target sedation goal in 50% of the patients. ED 95 was defined as the effective initial end-tidal sevoflurane concentration to achieve a target sedation goal in 95% of the patients.
After determining the success or failure in achieving the target sedation level by adjusting the concentration of sevoflurane, we adjusted the inhalation agent dose to maintain the depth of sedation corresponding to a RASS score of –2 to –3 while continuing mechanical ventilation. Pain was assessed using the CPOT score at least three times a day and reassessed after 30 minutes if any intervention that could affect the pain score of the patients was performed. The remifentanil infusion rate was adjusted to maintain a CPOT score of < 3. Both sedation and analgesia levels were monitored continuously by the patient’s nursing staff. The infusion dose was immediately adjusted by the attending ICU physician if needed. Based on the patient’s general condition and flap condition, the attending surgeon discussed the possibility of stopping sedation on a daily basis. After determining when the patient should be woken up, sevoflurane administration was stopped. Mechanical ventilation was stopped after confirming the patient’s appropriate level of consciousness and spontaneous breathing.
Statistical analysis
The ED 50 was calculated using Dixon’s up and down method. According to this method, our prospective study was ended when we found six pairs of successes and failures in achieving the sedation goal. Assuming that the concentration of sevoflurane was positively correlated with the response rate, the isotonic regression method was used to calculate the ED 50 and ED 95. The 95% confidence intervals (CIs) were derived using the bootstrap method [21,22].
Retrospective study
Patients
In a retrospective analysis, we compared the prospective study group with the IV propofol sedation group. The patients in the IV sedation group received postoperative sedation with IV propofol for the same reason as those in the volatile sevoflurane group between April 2018 and January 2019. The IV sedation group received the same anesthetic management as did the sevoflurane group during surgery. The protocols to maintain the sedation depth were the same between the two groups. We adjusted the propofol infusion dose to maintain the depth of sedation corresponding to a RASS score of –2 to –3 while continuing mechanical ventilation. Pain assessments were based on the CPOT scores. The remifentanil dose was adjusted to maintain a CPOT score of < 3. The nursing staff continuously monitored the sedation and analgesia levels. The infusion dose was immediately adjusted by the attending ICU physician if needed. The propofol infusion was stopped when the surgeon determined that the patient was stable to be awakened. Mechanical ventilation was stopped after confirming the patient’s level of consciousness and spontaneous breathing.
Data collection
We compared the following parameters between the two groups: intraoperative remifentanil infusion rate; postoperative remifentanil infusion rate while sevoflurane or propofol infusion was being administered to maintain mechanical ventilation; norepinephrine use while sevoflurane or propofol was being administered; the incidence of postoperative delirium until discharge diagnosed using the Confusion Assessment Method for the ICU and Intensive Care Delirium Screening Checklist; length of ICU stay, length of hospital stay; and incidence of serious adverse events, such as mortality, myocardial infarction, cerebral infarction, and kidney or liver failure, until discharge from the hospital.
Statistical analysis
The recorded and calculated parameters were described as means or medians (interquartile range). For the categorical data, we calculated absolute and relative frequencies (count and %). Intergroup comparisons were performed using the Student t-test or Mann-Whitney’s rank sum test (with Bonferroni correction), chi-square test, or Fisher’s test (complete response, %). The P-values < 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS ver. 23 (IBM Corp., Armonk, NY, USA) and R package, version 3.5.1 (R Foundation, Vienna, Austria).
RESULTS
Prospective Study
Twenty-five patients were enrolled. The median age was 62 years (54.5–70.5 years). The mean body mass index (BMI) was 23.2 ± 3.4 kg/m2. The median postoperative sedation duration was 680 minutes (570–815 minutes). Six pairs of success-failure were collected (Figure 1). The ED 50 of the initial end-tidal sevoflurane concentration was 0.40% ± 0.16% by the Dixon’s method. By the isotonic regression method, the ED 50 of sevoflurane was 0.36% (83% CI, 0.26% to 0.54%; 95% CI, 0.20 to 0.60%) and the ED 95 was 0.69% (83% CI, 0.62 to 0.72%; 95% CI, 0.60 to 0.75%) (Table 1, Figure 2). These optimal concentrations were achieved in the sedation protocol using remifentanil as an analgesic.
Retrospective Study
Forty-nine patients were enrolled in the retrospective study. We confirmed that the RASS (–2 to –3) and CPOT scores (< 3) were appropriately controlled according to the patient’s nursing records. Under the same level of sedation and analgesia, the postoperative remifentanil infusion rate was significantly lower in the sevoflurane group (2.52 ± 1.00 µg/kg/hr, P = 0.001) than it was in the IV sedation group (3.66 ± 1.30 µg/kg/hr).
The patients’ characteristics and operative events are described in Table 2. There were no significant differences in age, sex, BMI, underlying systemic disease, duration of surgery, or intraoperative remifentanil infusion rate between the two groups. The postoperative sedation duration of mechanical ventilation was longer in the propofol group than it was in the sevoflurane group, although this difference was not significant (P = 0.099). Serious adverse events, such as mortality, myocardial infarction, cerebral infarction, and kidney or liver failure, were not observed until discharge from the hospital in either group. Postoperative delirium was also not observed in either group. The length of ICU and hospital stays did not vary between the groups. Norepinephrine use during sedative agent infusion was also similar between the two groups.
DISCUSSION
In this prospective study, we determined the proper initial end-tidal concentration of sevoflurane for patients who underwent head and neck surgery with tracheostomy. The ED 50 of sevoflurane was 0.40% ± 0.16% by Dixon’s method. In contrast, the ED 50 using the isotonic regression method was 0.36% with an ED 95 of 0.69%. The main finding of our retrospective study is that there was a lower opioid requirement during mechanical ventilation in the sevoflurane group than there was in the IV sedation group. To the best of our knowledge, this is the first study on postoperative sevoflurane sedation in patients who underwent head and neck surgery with tracheostomy.
Complications such as airway obstruction, edema, bleeding, and aspiration are life-threatening factors after head and neck surgery. In addition, excessive movement and hemodynamic instabilities due to postoperative agitation have an adverse effect on flap survival. Therefore, adequate sedation and mechanical ventilation in the ICU immediately after surgery is the standard care in many institutions [23-25]. Head and neck surgery can also cause damage to the cervical blood vessels and nerves. Therefore, these patients should be intensively monitored for perioperative cerebrovascular accidents during sedation. It is essential to perform daily sedation interruptions to examine the patients’ neurologic status [26,27]. For these reasons, the sedative agent used for head and neck surgery should not only provide moderate or deep sedation, but also enable rapid awakening after interruption.
In order to achieve sedation that avoids purposeful movement in response to a supramaximal stimulus, the end-tidal concentration of the volatile agent should be slightly above the minimum alveolar concentration (MAC)-awake. The MAC-awake is usually approximately one-third of the MAC and is typically 0.6% for sevoflurane [19]. Considering this, the manufacturer recommends initiating administration with 0.5% of sevoflurane for sedation. Previous studies on applying the ACD to postoperative sedation after cardiac surgery followed that protocol [12,28]. However, in those studies, the airways were secured by orotracheal intubation and not by tracheostomy. We also need a moderate degree of sedation (RASS score –2 to –3) to prevent life threatening complications. In this study, the ED 95 of end-tidal sevoflurane was 0.69%. This difference may be related to a deeper sedation target, the method of securing the airway, and airway irritability due to surgery.
Various adverse events can occur during sedation in critically ill patients. Unpredictable pharmacokinetic and pharmacodynamic effects due to drug interactions, organ dysfunction, inconsistent absorption and protein binding, hemodynamic instability, and drug accumulation can cause serious adverse events. Therefore, setting up appropriate initial concentration for sedation is of utter importance for the safety of patients in the ICU. Based on our results, we can set the initial sevoflurane concentration for patients with tracheostomy. This can also help to develop standard clinical guidelines for sevoflurane postoperative sedation in Asian patients. To the best of our knowledge, this is the first study to investigate the initial sevoflurane concentration setting to sedate critically ill postoperative patients in Asia.
There were no significant differences in the length of ICU stay, hospital stay, or in vasoactive drug use during sedative agent administration between the sevoflurane and propofol groups. Moreover, no serious adverse events or delirium were noted in either group. In addition, the sevoflurane group had lower opioid use than did the propofol group during sedative agent infusion to maintain mechanical ventilation. We used a lower dose of opioids in the sevoflurane group while providing the same degree of sedation (RASS –2 to –3) and pain control (CPOT score of <3). Our findings are consistent with those of several prior studies. [14,29]. In another study, a subanesthetic level of sevoflurane was found to provide an analgesic effect in the sevoflurane group [30]. Opioid infusion can cause acute opioid tolerance and opioid-induced hyperalgesia. Therefore, for patients with higher opioid requirements, inhalation sedation can be used as an option to reduce opioid consumption [31,32].
Inhalation sedatives have some characteristics of ideal sedatives, such as quick sedation effect onset, rapid awakening after interruption, little accumulation after long-term administration, and little impact on clearance and elimination with regard to hepatic or renal function. In addition, propofol infusion syndrome can be avoided if an inhalation sedative is used. Our results suggest that inhalation sedation is a good alternative to IV sedation.
Our study has several limitations. We could not perform a prospective study to compare the sevoflurane and propofol groups. Further prospective randomized-controlled studies with larger patient groups can supplement this limitation. Furthermore, a double-blind trial was infeasible because of the different devices between the sevoflurane and propofol groups. An ACD requires a specific device to connect to the mechanical ventilator. This might have introduced bias by the medical staff that managed the enrolled patients. Another limitation is that midazolam is classified as a short-acting agent according to its metabolism and plasma clearance. It has a short distribution half-life and was injected evenly. Therefore, we assumed that it did not affect the initial sedation depth, but this possibility cannot be excluded [33]. Finally, this study was performed in a group of critically ill patients who underwent tracheostomy. Depending on the manner of securing the airway, the delivery amount of sevoflurane can vary and differ from the monitored level. Therefore, the monitored concentration in the tracheostomy group can differ from that of the endotracheal group. Further study is needed to determine the differences across different types of airways.
In conclusion, the ED 50 and ED 95 of the end-tidal sevoflurane concentration for optimal postoperative sedation in patients who underwent head and neck surgery with tracheostomy were 0.36% and 0.69%, respectively. There was no difference in the clinical outcomes between IV sedation using propofol and inhalation sedation using an ACD. The sevoflurane group demonstrated lower opioid use than did the IV sedation group during sedative agent infusion. Inhaled sevoflurane sedation using an ACD (AnaConDa) seems to be a favorable alternative option to conventional propofol sedation.
KEY MESSAGES
▪ We determined the proper initial end-tidal concentration of sevoflurane in postoperative patients with tracheostomies.
▪ This is the first study on postoperative sevoflurane sedation in patients who underwent head and neck surgery with tracheostomy.
▪ Postoperative inhalation sedation can reduce opioid consumption after surgery.
NOTES
-
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Acknowledgments
The research was supported by basic science research program through the NRF funded by the ministry of science and ICT (2017R1c1b5077169) to Jeongmin Kim.
The authors would like to thank Ms. Hye Jung Shin, a senior statistician (Biostatistics Collaboration Unit, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea) for her statistical help with the data analysis.
NOTES
-
AUTHOR CONTRIBUTIONS
Conceptualization: SN, HBK, JK. Data Curation: SJ, HBK, HJJ. Formal analysis: HJJ. Funding acquisition: JK. Methodology: SN. Project administration: JK. Visualization: SJ, SN. Writing–original draft: SJ. Writing–review & editing: SJ, JK.
Figure 1.Sevoflurane concentrations for sedation targeted at achieving a Richmond agitation-sedation scale score of –2 to –3 using the Dixon’s up-and-down method. ED 50 (ED95), the effective initial end-tidal sevoflurane concentration for achieving the target sedation goal in 50% (95%) of the patients.
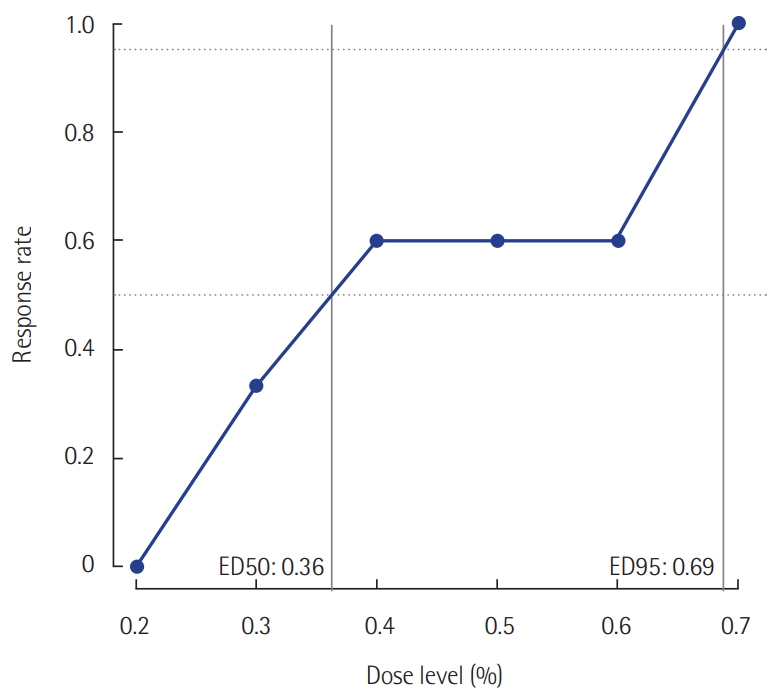
Figure 2.Response rates evaluated using the isotonic regression method.
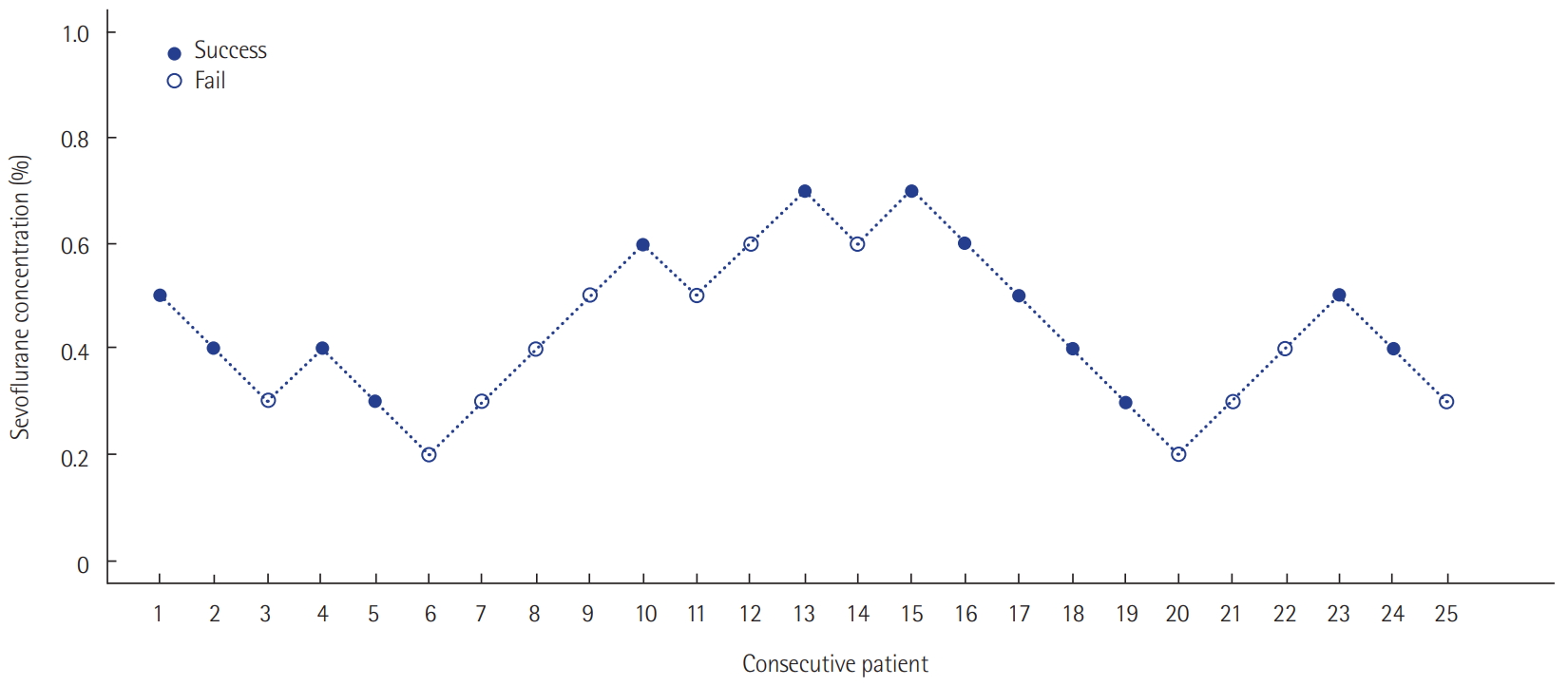
Table 1.Initial end-tidal sevoflurane concentration determined by Dixon’s method and the isotonic regression method
|
Variable |
Dixon’s method |
Isotonic regression method
|
|
ED50 |
ED95 |
|
Sevoflurane (%) |
0.40±0.16 |
0.36 |
0.69 |
|
(83% CI, 0.26–0.54; 95% CI, 0.20–0.60) |
(83% CI, 0.62–0.72; 95% CI, 0.60–0.75) |
Table 2.Demographics, perioperative data, and postoperative outcomes
|
Variable |
Sevoflurane group (n=25) |
Propofol group (n=24) |
P-valuea
|
|
Age (yr) |
62 (54.5–70.5) |
61 (57–65) |
0.609 |
|
Male sex |
18 (72) |
17 (70.8) |
0.928 |
|
Body mass index (kg/m2) |
23.2±3.4 |
23.3±3.8 |
0.908 |
|
ASA physical statusb
|
|
|
0.866 |
|
I |
9 (36) |
7 (29) |
|
|
II |
8 (32) |
9 (38) |
|
|
III |
8 (32) |
8 (33) |
|
|
Duration of surgery (min) |
702.6±179.8 |
659.2±248.4 |
0.485 |
|
Duration of postoperative sedation |
771.0±388.4 |
1,508.2±2,074.7 |
0.099 |
|
Intraoperative remifentanil infusion rate (µg/kg/hr) |
3.52±1.00 |
3.47±1.17 |
0.883 |
|
Postoperative remifentanil infusion rate during sedative agent infusion (µg/kg/hr) |
2.52±1.00 |
3.66±1.30 |
0.001 |
|
ICU stay (day) |
2 (2–2) |
2 (2–2) |
0.208 |
|
Hospital stay (day) |
22.8±7.2 |
26.4±12.6 |
0.226 |
|
Delirium during hospital stay |
0 |
0 |
|
|
Fluid balancec (ml/hr) |
|
|
|
|
Day 1 |
37.9±38.3 |
29.7±38.5 |
0.457 |
|
Day 2 |
8.8±37.7 |
11.2±34.1 |
0.813 |
|
Number of patients receiving norepinephrine during sedative drug administration |
11/25 (44) |
12/24 (50) |
0.674 |
|
Norepinephrine infusion time during sedative drug administration (min) |
1,026.8±1,742.1 |
1,907.9±2,784.5 |
0.379 |
References
- 1. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018;46:e825-e73.ArticlePubMed
- 2. Kovatch KJ, Hanks JE, Stevens JR, Stucken CL. Current practices in microvascular reconstruction in otolaryngology-head and neck surgery. Laryngoscope 2019;129:138-45.ArticlePubMed
- 3. Marsh M, Elliott S, Anand R, Brennan PA. Early postoperative care for free flap head & neck reconstructive surgery: a national survey of practice. Br J Oral Maxillofac Surg 2009;47:182-5.ArticlePubMed
- 4. Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: a systematic review. JAMA 2000;283:1451-9.ArticlePubMed
- 5. Westcott C. The sedation of patients in intensive care units: a nursing review. Intensive Crit Care Nurs 1995;11:26-31.ArticlePubMed
- 6. Shafer A. Complications of sedation with midazolam in the intensive care unit nd a comparison with other sedative regimens. Crit Care Med 1998;26:947-56.ArticlePubMed
- 7. Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth 1998;8:491-9.ArticlePubMed
- 8. Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia 2007;62:690-701.ArticlePubMed
- 9. Mirrakhimov AE, Voore P, Halytskyy O, Khan M, Ali AM. Propofol infusion syndrome in adults: a clinical update. Crit Care Res Pract 2015;2015:260385. ArticlePubMedPMCPDF
- 10. Purrucker JC, Renzland J, Uhlmann L, Bruckner T, Hacke W, Steiner T, et al. Volatile sedation with sevoflurane in intensive care patients with acute stroke or subarachnoid haemorrhage using AnaConDa®: an observational study. Br J Anaesth 2015;114:934-43.ArticlePubMedPDF
- 11. Soukup J, Schärff K, Kubosch K, Pohl C, Bomplitz M, Kompardt J. State of the art: sedation concepts with volatile anesthetics in critically Ill patients. J Crit Care 2009;24:535-44.ArticlePubMed
- 12. Röhm KD, Wolf MW, Schöllhorn T, Schellhaass A, Boldt J, Piper SN. Short-term sevoflurane sedation using the Anaesthetic Conserving Device after cardiothoracic surgery. Intensive Care Med 2008;34:1683-9.ArticlePubMedPDF
- 13. Migliari M, Bellani G, Rona R, Isgrò S, Vergnano B, Mauri T, et al. Short-term evaluation of sedation with sevoflurane administered by the anesthetic conserving device in critically ill patients. Intensive Care Med 2009;35:1240-6.ArticlePubMedPDF
- 14. Mesnil M, Capdevila X, Bringuier S, Trine PO, Falquet Y, Charbit J, et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med 2011;37:933-41.ArticlePubMedPDF
- 15. Farrell R, Oomen G, Carey P. A technical review of the history, development and performance of the anaesthetic conserving device “AnaConDa” for delivering volatile anaesthetic in intensive and post-operative critical care. J Clin Monit Comput 2018;32:595-604.ArticlePubMedPMCPDF
- 16. Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol 2017;112:18-35.ArticlePubMedPDF
- 17. Patel PM, Drummond JC, Lemkuil BP. Cerebral physiology and the effects of anesthetic drugs. In: Miller RD. In: Miller’s Anesthesia. 8th ed. Philadelphia, Elsevier Saunders. 2015, pp 387-422.
- 18. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44.ArticlePubMed
- 19. Meiser A, Laubenthal H. Inhalational anaesthetics in the ICU: theory and practice of inhalational sedation in the ICU, economics, risk-benefit. Best Pract Res Clin Anaesthesiol 2005;19:523-38.ArticlePubMed
- 20. Meiser A, Bellgardt M, Belda J, Röhm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anaesthetic conserving device: a bench study with isoflurane and sevoflurane. J Clin Monit Comput 2009;23:11-9.ArticlePubMedPMCPDF
- 21. Dilleen M, Heimann G, Hirsch I. Non-parametric estimators of a monotonic dose-response curve and bootstrap confidence intervals. Stat Med 2003;22:869-82.ArticlePubMed
- 22. Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics 2002;58:171-7.ArticlePubMed
- 23. Godden DR, Patel M, Baldwin A, Woodwards RT. Need for intensive care after operations for head and neck cancer surgery. Br J Oral Maxillofac Surg 1999;37:502-5.ArticlePubMed
- 24. Manrique OJ, Sabbagh MD, Kapoor T, Ciudad P, Chen HC. Postoperative management after total pharyngolaryngectomy using the free ileocolon flap: a 5-year surgical intensive care unit experience. Ann Plast Surg 2020;84:68-72.ArticlePubMed
- 25. Varadarajan VV, Arshad H, Dziegielewski PT. Head and neck free flap reconstruction: what is the appropriate post-operative level of care? Oral Oncol 2017;75:61-6.ArticlePubMed
- 26. Nosan DK, Gomez CR, Maves MD. Perioperative stroke in patients undergoing head and neck surgery. Ann Otol Rhinol Laryngol 1993;102:717-23.ArticlePubMed
- 27. Rechtweg J, Wax MK, Shah R, Granke K, Jarmuz T. Neck dissection with simultaneous carotid endarterectomy. Laryngoscope 1998;108(8 Pt 1):1150-3.ArticlePubMed
- 28. Prieto Vera CJ, del Cojo Peces E, Macías Pingarrón JP, Asencio Moreno A, Andújar Quirós B, Gragera Collado I. Anesthetic conserving device (AnaConDa) used after cardiac surgery: experience in a postoperative recovery unit. Rev Esp Anestesiol Reanim 2011;58:421-5.ArticlePubMed
- 29. Lin CK, Feng YT, Hwang SL, Lin CL, Lee KT, Cheng KI. A comparison of propofol target controlled infusion-based and sevoflurane-based anesthesia in adults undergoing elective anterior cervical discectomy and fusion. Kaohsiung J Med Sci 2015;31:150-5.ArticlePubMed
- 30. Yeo ST, Holdcroft A, Yentis SM, Stewart A. Analgesia with sevoflurane during labour: i. Determination of the optimum concentration. Br J Anaesth 2007;98:105-9.ArticlePubMedPDF
- 31. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104:570-87.ArticlePubMed
- 32. Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008;24:479-96.ArticlePubMed
- 33. Vuyk J, Sitsen E, Reekers M. Intravenous anesthetics. In: Miller RD. In: Miller’s anesthesia. 8th ed. Philadelphia, Elsevier Saunders. 2015, pp 821-63.
Citations
Citations to this article as recorded by

- Halogenated anesthetics vs intravenous hypnotics for short and long term sedation in the intensive care unit: A meta-analysis
V. Likhvantsev, G. Landoni, N. Ermokhina, M. Yadgarov, L. Berikashvili, K. Kadantseva, O. Grebenchikov, L. Okhinko, A. Kuzovlev
Medicina Intensiva.2023; 47(5): 267. CrossRef - Halogenated anesthetics vs intravenous hypnotics for short and long term sedation in the intensive care unit: A meta-analysis
V. Likhvantsev, G. Landoni, N. Ermokhina, M. Yadgarov, L. Berikashvili, K. Kadantseva, O. Grebenchikov, L. Okhinko, A. Kuzovlev
Medicina Intensiva (English Edition).2023; 47(5): 267. CrossRef - Inhaled Sedation with Volatile Anesthetics for Mechanically Ventilated Patients in Intensive Care Units: A Narrative Review
Khaled Ahmed Yassen, Matthieu Jabaudon, Hussah Abdullah Alsultan, Haya Almousa, Dur I Shahwar, Fatimah Yousef Alhejji, Zainab Yaseen Aljaziri
Journal of Clinical Medicine.2023; 12(3): 1069. CrossRef - Sedation with Sevoflurane versus Propofol in COVID-19 Patients with Acute Respiratory Distress Syndrome: Results from a Randomized Clinical Trial
Sara Martínez-Castro, Berta Monleón, Jaume Puig, Carolina Ferrer Gomez, Marta Quesada, David Pestaña, Alberto Balvis, Emilio Maseda, Alejandro Suárez de la Rica, Ana Monero Feijoo, Rafael Badenes
Journal of Personalized Medicine.2023; 13(6): 925. CrossRef - Effect of inhaled anaesthetics on cognitive and psychiatric outcomes in critically ill adults: a systematic review and meta-analysis
Sean Cuninghame, Angela Jerath, Kevin Gorsky, Asaanth Sivajohan, Conall Francoeur, Davinia Withington, Lisa Burry, Brian H. Cuthbertson, Beverley A. Orser, Claudio Martin, Adrian M. Owen, Marat Slessarev, Martin Chapman, Damon Scales, Julie Nardi, Beth Li
British Journal of Anaesthesia.2023; 131(2): 314. CrossRef - Experiencia y revisión de la literatura del uso del dispositivo Anesthetic Conserving Device (AnaConDa) durante la pandemia en pacientes con neumonía por COVID-19 en un hospital público
María Guadalupe Morales Hernández, Marcelo Díaz Conde, Ixchel Magaña Matienzo
Medicina Crítica.2023; 37(4): 334. CrossRef - Sedation of patients in intensive care units. Guidelines
V.I. Potievskaya, I.B. Zabolotskikh, I.E. Gridchik, A.I. Gritsan, A.A. Eremenko, I.A. Kozlov, A.L. Levit, V.A. Mazurok, I.V. Molchanov
Anesteziologiya i reanimatologiya.2023; (5): 6. CrossRef - Inhaled volatile anesthetics in the intensive care unit
Erin D Wieruszewski, Mariam ElSaban, Patrick M Wieruszewski, Nathan J Smischney
World Journal of Critical Care Medicine.2023;[Epub] CrossRef - Prospects of inhalation sedation in intensive care
O.A. Grebenchikov, V.V. Kulabukhov, A.K. Shabanov, O.V. Ignatenko, V.V. Antonova, R.A. Cherpakov, I.V. Redkin, E.A. Boeva, A.N. Kuzovlev
Anesteziologiya i reanimatologiya.2022; (3): 84. CrossRef - Análisis nacional de la sedación aplicada en pacientes de cuidados críticos
Grace Pamela López Pérez, Melani Dayana Carrera Casa, Gissela Lizbeth Amancha Moyulema, Yadira Nathaly Chicaiza Quilligana, Ana Belén Guamán Tacuri, Joselyn Mireya Iza Arias
Salud, Ciencia y Tecnología.2022; 2(S1): 234. CrossRef
 , Sungwon Na
, Sungwon Na , Hye Bin Kim
, Hye Bin Kim , Hye Ji Joo
, Hye Ji Joo , Jeongmin Kim
, Jeongmin Kim




 KSCCM
KSCCM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite