Abstract
- Visual complications represent common deficits following surgical or endovascular repair of paraclinoid aneurysms. Different etiologies should be investigated to prevent devastating consequences. Herein we present a point-of-care evaluation to investigate sudden visual loss after coiling of paraclinoid aneurysms. A 20-year-old male was admitted for a sudden headache. Head computed tomography showed a subarachnoid hemorrhage and subsequent angiography revealed a 9-mm left supraclinoid aneurysm of the internal carotid artery treated with endovascular coil embolization. Thirty minutes after intensive care unit admission, the patient reported a left amaurosis. To exclude secondary etiologies, an immediate evaluation with point-of-care devices (color-doppler and B-mode ultrasound and automated pupillometry) was performed. Sonographic evaluations were negative for ischemic/thrombotic events and neurologic pupil index within physiological ranges provide evidence of third cranial nerve responsiveness. The symptomatology resolved progressively over 120 minutes with low-dose steroid therapy, 30° head-of-bed elevation, and blood pressure management. Visual deficits can occur after endovascular procedure and should be investigated. Suspected visual loss is a neurological emergency that deserves a prompt evaluation. Ultrasound and automated pupillometry have proved to be an effective, rapid, reliable, and non-invasive combination for a clinical decision-making strategy in the management of post-procedural acute visual deficits.
-
Keywords: amaurosis fugax; point-of-care; pupillometry; subarachnoid hemorrhage; ultrasound
Patients harboring aneurysms in the ophthalmic and paraclinoid segment of the internal carotid artery (ICA) frequently present with visual impairment, given the close anatomical relationship between the ophthalmic artery and the optic nerve. The visual impairment may be the presenting symptom, of direct compression, or iatrogenic event following the surgical or endovascular treatment [1]. The knowledge of possible complications and adverse effects is crucial among physicians facing this pathology and the ocular status should be promptly evaluated in case of new or worsened visual deficits. Color-doppler and automated pupillometry are useful point-of-care tools, associated with the neuro-ophthalmological examination, for the evaluation of the patients complaining new visual deficits and for the monitoring of unconscious patients. We present an exemplificative case of a patient suffering an aneurysmal subarachnoid hemorrhage (aSAH) who developed an isolated visual loss after the endovascular procedure to describe a practical bedside screening technique for the evaluation of new-onset visual deficits.
CASE REPORT
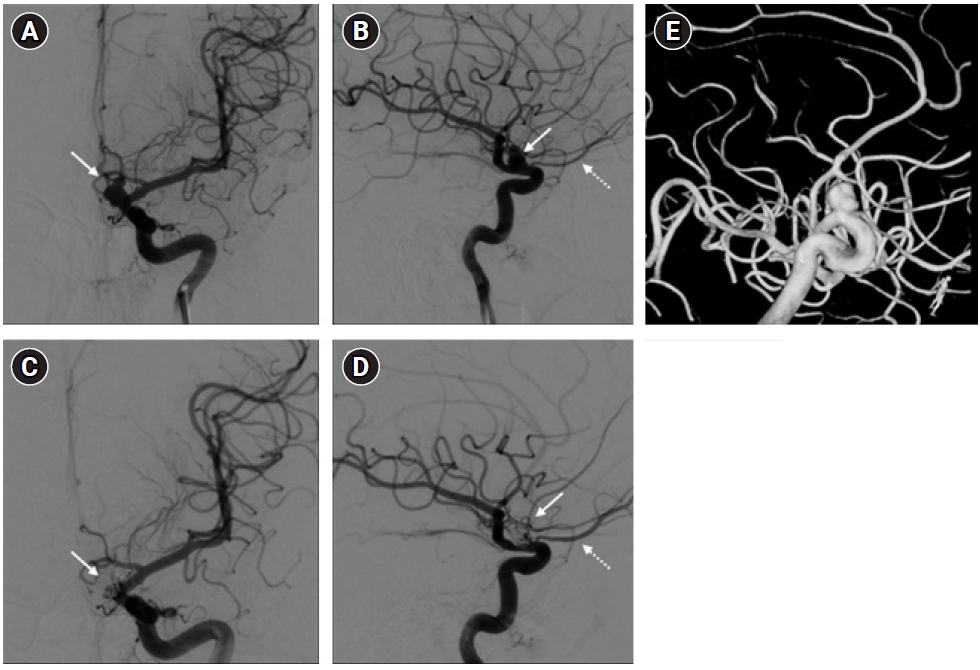
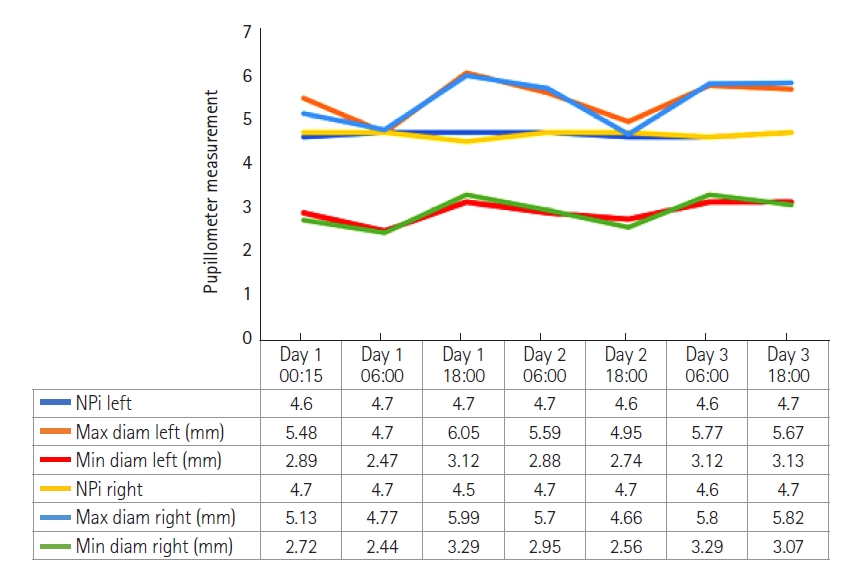
A 20-years-old male, with a silent medical history apart from a smoking habit, overnight experienced a sudden headache and was admitted to the emergency department. A brain computed tomography (CT) scan showed a suspected aSAH (modified Fisher score 1). The clinical condition was unremarkable apart from a moderate headache (Glasgow coma scale, 15; World Federation of Neurosurgical Societies grade, 1; Hunt-Hess grade, 2). The subsequent cerebral angiography confirmed the presence of a 9-mm left ophthalmic/paraclinoid irregular aneurysm (Figure 1A, B, and E). The aneurysm was secured by an endovascular coiling procedure. After the embolization, the aneurysm exclusion and the patency of the ophthalmic artery were confirmed by new angiographic sequences (Figure 1C and D). Thirty minutes after the intensive care unit admission, during a routinely neurological assessment, the patient presented a painless, acute left mono-lateral visual loss (monocular blindness) in the absence of focal neurological or cranial nerve deficits (consensual pupillary light reflex). Supported by continuous monitoring of general vital signs and neurological examination, a rapid and specific neurocritical evaluation was performed with the aid of specific point-of-care tools. Initially, the optic nerve sheath diameter (ONSD) of both eyes was assessed according to the CLOSED protocol [2]; B-mode ultrasound measurements of the ONSD ruled out intracranial hypertension as normal and symmetrical values were observed (3.5 mm and 3.6 mm for the left and right sagittal diameter, respectively) (Figure 2A and B). As further test, the ocular vessel patency was confirmed by the ultrasound color-doppler mode, excluding large vessel occlusion, thrombo-embolic affections of the retinal blood supply, and early vasospasm. The physiological flow and shape of the ophthalmic artery were reported in Figure 2C and D. Finally, an automated pupillometry was used to assess the pupillary function and the third cranial nerve status. The neurologic pupil index (NPi) was determined and resulted within physiological ranges in both eyes (4.6 and 4.7 in the left and right eye, respectively). Finally, a standard ophthalmoscopic exam including fundus, retinal vessels, and optic disk evaluation excluded embolic phenomena and papilledema. Given the absence of objective clinical causes that could explain the presenting symptoms, a low-dose steroid therapy infusion, a 30° head-of-bed position and a stable mean arterial pressure were the conduct of choice. The visual field had primarily reappeared in the lower quadrants after 60 minutes and the entire symptomatology gradually resolved over the following 120 minutes. The neurological status remained stable in the following days, with progressive restoration of binocular vision, leading to the diagnosis of amaurosis fugax. The pupillometer measurement was then repeated several times in the following days (mean value±standard deviation: NPi left, 4.6±0.1; NPi right, 4.7±0.1), to confirm the stability of the detected data (Figure 3). The endovascular treatment of the aneurysm was completed a few weeks later using a flow-diverter stent with an uneventful course.
DISCUSSION
Sudden visual loss is a medical emergency that requires a prompt evaluation and treatment. Aneurysm arising near the ophthalmic segment of the ICA could represent a clinical challenge as their treatment is burdened by new visual disturbance or worsening of previous visual symptoms in up to 28.5% of patients [1,3-5]. Compressive, manipulative and thermal injuries to the optic nerve represent the most frequent causes of postoperative visual deficits related to the surgical treatment [4,6-8]. Conversely, mass effect, ischemic event and post-procedural swelling related to perianeurysmal inflammation processes after the endovascular treatment have been theorized as a possible mechanism responsible for the onset or worsening of visual deficits after endovascular procedure, with a 6.4% rate of permanent visual disturbances [9,10]. Therefore, physicians who routinely manage aSAH in the clinical practice should be aware of this complication and the techniques for an appropriate management, in order to provide a prompt and targeted response to deal with this emergency. This case offers the opportunity to describe an in-depth evaluation of the patients who experienced a sudden visual loss, or the evaluation of unconscious patients, within a few minutes at the patient's bed-side providing useful data in the detection of the problem and leading the subsequent treatment. The exclusion of any potential causes of intracranial hypertension and time-dependent pathology should be mandatory at the occurrence of a new neurological symptom. Despite the neurological evaluation remaining the cornerstone exam, the sonographic measurements of the ONSD could provide indirect esteem of the intracranial pressure (ICP) [11] directing those patients who could benefit from further analysis (e.g., brain CT) in case of pathological values or doubtful clinical scenarios. Once ruled out an increase in the ICP, the sonographic device could be easily switched from the B-mode to the color-doppler mode for the ocular vessel examination [2]. Frequently visual loss could be secondary to spontaneous or provoked embolic events in the ophthalmic vessels or vasospasm. Even in this case, the sonographic examination has proven to be a reliable tool in the recognition of these types of phenomena [12,13]. In fact, the correct visualization of the ophthalmic artery, the central retinal artery, and the central retinal vein, in the absence of flow obstruction and spot sign as in our case, confirm the vessel patency and averts the need for thrombolytic therapy [14]. Despite those applications, the sonographic assessment is burdened by some drawbacks such as high inter-/intra-observer variability, potential artifacts, and the timing to reach an adequate learning curve [15].
Another useful point-of-care device in the ophthalmic evaluation is represented by automated pupillometry. Emerging evidence suggests that pupillometry could be a suitable tool for monitoring and detecting early variation in the neurological status, such as ICP rising, recognition of delayed cerebral ischemia, estimating the clinical severity of aSAH and discrimination between compressive and ischemic events in the third nerve palsy [16-18]. However, even in this case, limitations of this technology related to potential confounders on NPi such as medications, pain, facial and/or ocular injuries, and elevated ICP should be considered [19]. In the present case, the pupillometry investigation was within the physiological range, ruling out microvascular ischemic damage (decreased maximum and minimum pupil size) [18], increased ICP (NPi decrease to <3) [20], delayed cerebral ischemia (NPi decrease to <3) [16], and possible compression on the ipsilateral third cranial nerve (decrease in pupillary constriction velocities) [18] in the acute presentation. In the light of those findings, we hypothesize that the visual deficits might be attributed to a hypoperfusive status secondary to aSAH-induced cerebral dysautoregulation or transient microembolic vascular occlusion not detectable at the ultrasound examination. The negative results of the carried-out examinations led to medical management of the symptoms, focused on the increase of the cerebral perfusion pressure, suppressing inflammation, and promoting followed by a complete clinical regression. As presented, in a short range of time the physician was able to face a visual loss complication, supported by objective and repeatable data, and to guide any further diagnostic tests in case of positive findings. Ultrasound and automated pupillometry have proved to be an effective, rapid, reliable, and non-invasive combination for a clinical decision-making strategy in the management of a post-procedural acute visual deficits.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conceptualization: GB, EM, RA. Data curation: RA. Formal analysis: GB, RA. Methodology: EM, RA. Project administration: GB, RA. Visualization: GB, RA. Writing–original draft: GB, EM, RA. Writing–review & editing: DM, CB.
Acknowledgments
Part of the manuscript was presented at the 18th annual meeting of the Neurocritical Care Society in September 22–25, 2020, in Phenix, USA.
Figure 1.Digital subtraction angiography of the head in the anteroposterior (A, C) and lateral (B, D) views of the left internal carotid artery. The ophthalmic aneurysm (arrows) and the left ophthalmic artery (dotted arrow) were shown in the pre-procedural images (A, B) and at the three-dimensional rotation angiography (E). The post-embolization images (C, D) showed the obliteration of the aneurysm (arrows) and the patency of the ophthalmic artery (dotted arrow).
Figure 2.Sonographic evaluation of the patient. The B-mode sonographic optic nerve sheath diameter assessment in the right (A; 3.6 mm) and left (B; 3.5 mm) eye excluded the presence of an elevated intracranial pressure and the absence of the hyperechogenic “spot sign.” The patency of the ophthalmic artery, with its characteristic dicrotic notch, was confirmed by the sonographic color-doppler evaluation in the right (C) and left (D) eye.
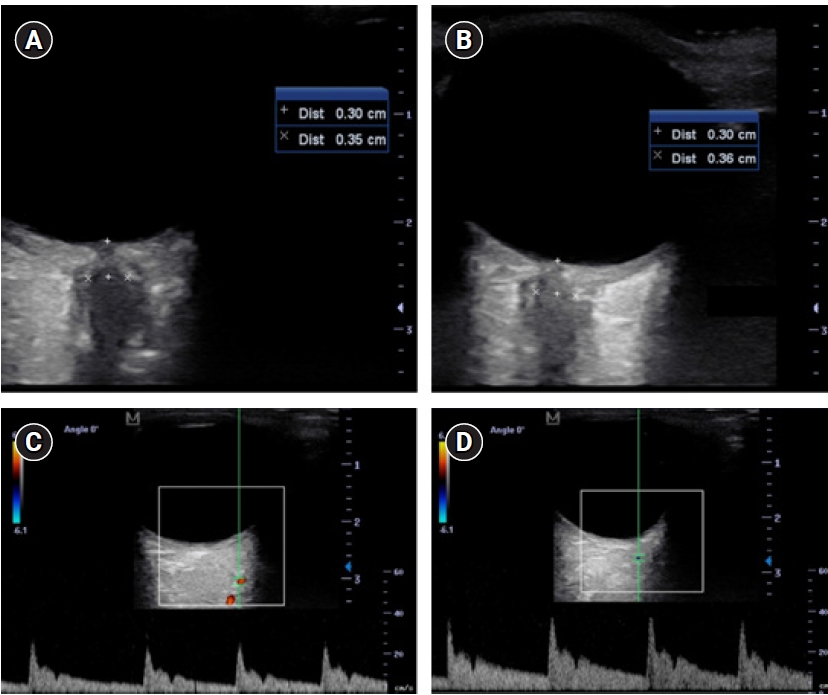
Figure 3.Graph showing detailed quantitative pupillometry results over time during the intensive care unit stay. The neurologic pupil index (NPi) is reported as an absolute number and the pupillary diameters in millimeter. Diam: diameter; Max: maximum; Min: minimum.
References
- 1. Silva MA, See AP, Dasenbrock HH, Patel NJ, Aziz-Sultan MA. Vision outcomes in patients with paraclinoid aneurysms treated with clipping, coiling, or flow diversion: a systematic review and meta-analysis. Neurosurg Focus 2017;42:E15. Article
- 2. Aspide R, Bertolini G, Albini Riccioli L, Mazzatenta D, Palandri G, Biasucci DG. A proposal for a new protocol for sonographic assessment of the optic nerve sheath diameter: The CLOSED protocol. Neurocrit Care 2020;32:327-32.ArticlePubMedPDF
- 3. Durst CR, Starke RM, Gaughen J, Nguyen Q, Patrie J, Jensen ME, et al. Vision outcomes and major complications after endovascular coil embolization of ophthalmic segment aneurysms. AJNR Am J Neuroradiol 2014;35:2140-5.ArticlePubMedPMC
- 4. Kamide T, Tabani H, Safaee MM, Burkhardt JK, Lawton MT. Microsurgical clipping of ophthalmic artery aneurysms: surgical results and visual outcomes with 208 aneurysms. J Neurosurg 2018;129:1511-21.ArticlePubMed
- 5. Kamide T, Burkhardt JK, Tabani H, Safaee M, Lawton MT. Microsurgical clipping techniques and outcomes for paraclinoid internal carotid artery aneurysms. Oper Neurosurg (Hagerstown) 2020;18:183-92.ArticlePubMedPDF
- 6. Date I, Asari S, Ohmoto T. Cerebral aneurysms causing visual symptoms: their features and surgical outcome. Clin Neurol Neurosurg 1998;100:259-67.ArticlePubMed
- 7. Horiuchi T, Goto T, Tanaka Y, Kodama K, Tsutsumi K, Ito K, et al. Role of superior hypophyseal artery in visual function impairment after paraclinoid carotid artery aneurysm surgery. J Neurosurg 2015;123:460-6.ArticlePubMed
- 8. Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N, et al. Risk factors for visual impairments in patients with unruptured intradural paraclinoid aneurysms treated by neck clipping without bypass surgery. World Neurosurg 2016;91:183-9.ArticlePubMed
- 9. Heran NS, Song JK, Kupersmith MJ, Niimi Y, Namba K, Langer DJ, et al. Large ophthalmic segment aneurysms with anterior optic pathway compression: assessment of anatomical and visual outcomes after endosaccular coil therapy. J Neurosurg 2007;106:968-75.ArticlePubMed
- 10. Michishita S, Ishibashi T, Yuki I, Urashima M, Karagiozov K, Kodama T, et al. Visual complications after coil embolization of internal carotid artery aneurysms at the ophthalmic segment. Interv Neuroradiol 2021;27:622-30.ArticlePubMedPMCPDF
- 11. Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care 2011;15:506-15.ArticlePubMedPDF
- 12. Foroozan R, Savino PJ, Sergott RC. Embolic central retinal artery occlusion detected by orbital color Doppler imaging. Ophthalmology 2002;109:744-7.ArticlePubMed
- 13. Ertl M, Altmann M, Torka E, Helbig H, Bogdahn U, Gamulescu A, et al. The retrobulbar "spot sign" as a discriminator between vasculitic and thrombo-embolic affections of the retinal blood supply. Ultraschall Med 2012;33:E263-7.ArticlePubMed
- 14. Mac Grory B, Lavin P, Kirshner H, Schrag M. Thrombolytic therapy for acute central retinal artery occlusion. Stroke 2020;51:687-95.ArticlePubMed
- 15. Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, et al. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med 2018;44:1284-94.ArticlePubMedPDF
- 16. Aoun SG, Stutzman SE, Vo PN, El Ahmadieh TY, Osman M, Neeley O, et al. Detection of delayed cerebral ischemia using objective pupillometry in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 2019;132:27-32.ArticlePubMed
- 17. Phillips SS, Mueller CM, Nogueira RG, Khalifa YM. A systematic review assessing the current state of automated pupillometry in the neuroICU. Neurocrit Care 2019;31:142-61.ArticlePubMedPDF
- 18. Kim HM, Yang HK, Hwang JM. Quantitative analysis of pupillometry in isolated third nerve palsy. PLoS One 2018;13:e0208259.ArticlePubMedPMC
- 19. Opic P, Rüegg S, Marsch S, Gut SS, Sutter R. Automated quantitative pupillometry in the critically ill: a systematic review of the literature. Neurology 2021;97:e629-42.ArticlePubMed
- 20. Chen JW, Gombart ZJ, Rogers S, Gardiner SK, Cecil S, Bullock RM. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the neurological pupil index. Surg Neurol Int 2011;2:82. ArticlePubMedPMC
Citations
Citations to this article as recorded by

 , Ernesto Migliorino3
, Ernesto Migliorino3 , Diego Mazzatenta1,2
, Diego Mazzatenta1,2 , Carlo Bortolotti2
, Carlo Bortolotti2 , Raffaele Aspide4
, Raffaele Aspide4









 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite