Abstract
- Invasive mechanical ventilation is a frequent therapy in critically ill patients in critical care units. To achieve favorable outcomes, patient and ventilator interaction must be adequate. However, many clinical situations could attempt against this principle and generate a mismatch between these two actors. These asynchronies can lead the patient to worst outcomes; that is why it is vital to recognize and treat these entities as soon as possible. Early detection and recognition of the different asynchronies could favor the reduction of the days of mechanical ventilation, the days of hospital stay, and intensive care and improve clinical results.
-
Keywords: asynchronies; detection; mechanical ventilation; recognition; treatment strategies
INTRODUCTION
Mechanical ventilation (MV) is one of the most used life supports in intensive care units (ICUs) [1]. Its main objectives are to maintain adequate gas exchange, decrease work of breathing or rest the ventilatory muscles until the patient's clinical condition is resolved or in the process of being resolved. Administering MV is not similar to administering a drug: interaction is much more complex and depends on multiple variables, some related to the patient (effort, demand, breathing timing) and others depend on the ventilator (trigger, flow, volumes). An optimal balance between these variables is what allows an adequate "patient-ventilator" synchrony. Mismatch between the patient's demand and the ventilator's delivery (in any of its phases) is defined as asynchrony.
Ventilator waveforms provide a continuous flow of information on patient-ventilator physiology and interaction (Figure 1). This information is invaluable for the detection and treatment of asynchronies since it has been reported its association with worse results [2]. The presence of ineffective efforts and double shots, for instance, has been shown to be related to mortality, longer MV and longer ICU stay [3]. Given the report of a new type of asynchrony [4], the proposal of this text is to develop a practical and concise clinical guide on the characteristics, causes, detection and strategies to prevent the deleterious effects of this entities. Although we did not conduct a formal systematic review, we do include studies that provide guidance on pathophysiology and clinical decision making.
GLOSSARY
Trigger
This parameter indicates the beginning of inspiration and ventilatory assistance. It is produced by the patient through inspiratory effort, generating synchrony in MV. Otherwise, alterations in trigger activation will cause a dyssynchrony in the MV-patient interaction [5-7].
Neural Inspiratory Time
It refers to the time in which diaphragmatic electrical activation (EAdi) occurs, specifically it is the time difference between the beginning of EAdi and the peak of inspiratory EAdi. It simply mentions the necessary time of the inspiratory phase according to the patient's respiratory demand and respiratory drive [8-11].
Mechanical or Ventilatory Inspiratory Time
The term indicates the time lapse of the programmed inspiratory phase in MV and tidal volume (TV). It is programmed according to expiratory time, respiratory rate (RR) and inspiratory to expiratory ratio [8-11].
Cycling
It represents the end of the inspiratory phase and the beginning of the passive expiratory phase [5,12].
Respiratory Drive
It is a physiological variable that refers to intensity of activity in respiratory centers, culminating on the activation and contraction of inspiratory muscles to generate a certain inspiratory flow. There are determinants that this impulse such as cortical, chemical and metabolic feedback, all present in critically ill patients [13-16].
Ventilatory Pressure
It is part of the equation of movement in MV:
Pmus+Pvent=E × V + R × F.
, where Pmus means muscle pressure; Pvent, ventilatory pressure; E, elastance; V, volume; R, resistance; and F, flow.
It refers to the work done by the mechanical ventilator, that is, the pressure generated in the respiratory system and its resistance and elastance properties to generate a certain volume and flow [17].
Muscle Pressure
Like Pvent, it is part of the equation of movement with the same purpose, however it is represented not by the work of the mechanical ventilator, but by the inspiratory muscles. During MV , individual participation, predominance or balance between Pvent and Pmus occurs [17].
ASYNCHRONY INDEX
Varon et al. [18] defined the asynchrony index (AI) as the percentage of monitored breaths that fail to be triggered, while Thille et al. [19] considered it as the number of events divided by the total RR, calculated as the sum of the number of ventilator cycles (activated or not) and ineffective shots. According to the above, this equation would be as follows.
AI=number of asynchrony events/total RR (ventilation cycles+lost efforts)×100.
They determine a high incidence of asynchrony as an AI >10%. One in four patients had a high incidence of asynchronies, the most common being IT and double trigger (DT), these patients were associated with longer ventilation times and a higher incidence of tracheostomy [19].
OUTCOMES
Many authors have studied the relationship between asynchronies and outcomes. Blanch et al. [20] found that patients who had an AI >10% had higher mortality rates, both in the ICU and hospital, and a tendency to greater duration of MV. Similar findings were published by Thille et al. [19], who also associated an AI with more days of MV.
In 2019, a group of researchers carried out a review that analyzed the results described in 16 studies, in which, apparently, high rates of asynchronies were associated with worse results. Due to the paucity of controlled trials, inconsistency between studies (on the quantification of the rate of asynchrony), the heterogeneity of the results and the paucity of patients recruited, the authors could not be conclusive about that association [2]. These findings coincide with the data recently published by Kyo et al. [21], where they showed that the number of asynchronies represented by the AI ≥10% may be associated with hard outcomes, such as the duration of MV, hospital and ICU mortality.
CLASSIFICATION
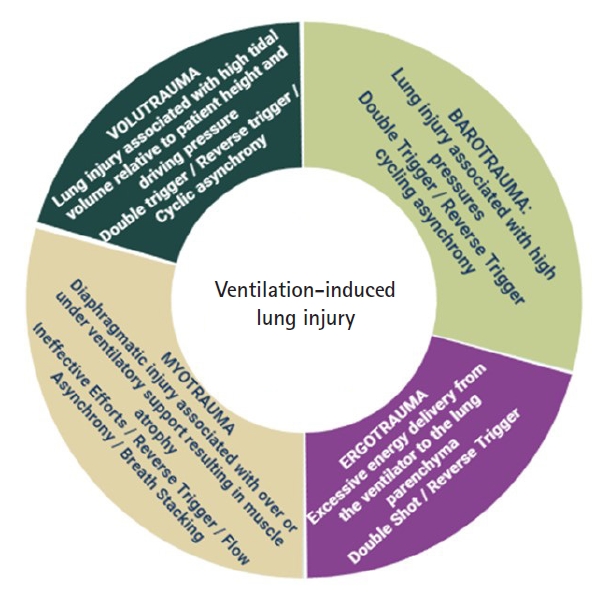
Different classification of asynchronies have been reported. According to Pham et al. [17], they can be grouped according to ventilatory assistance, distinguishing between over-assistance or low respiratory drive asynchrony and under-assistance or high respiratory drive asynchrony. Other authors [3,22,23] classify them according to the ventilatory cycle phase or divide them into clusters. With the aim of developing a friendly guide (Figure 2), a text is proposed that addresses each term in a brief and concise way, to facilitate its comprehension and provide different solutions.
TRIGGER ASYNCHRONIES
Reverse Trigger
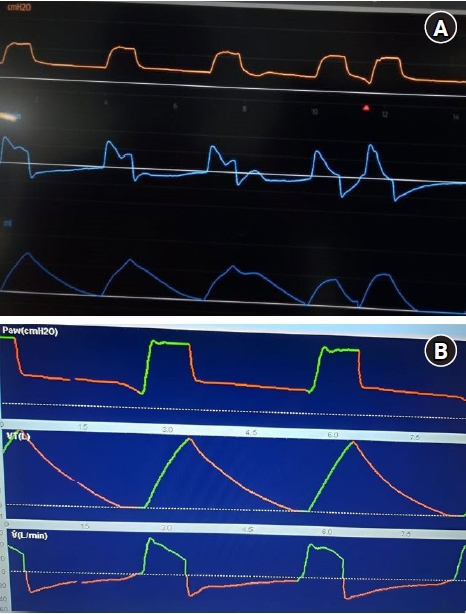
Reverse trigger (RT) described by Akoumianaki et al. [24], refers to the abnormal relation between the MV and diaphragmatic activation, where the external stimulus, the ventilator, provokes a response, that is, diaphragmatic contraction. It can be displayed as two continuous breathings without enough expiratory time, the first one is mandatory by the MV while the second by reflex contraction of the diaphragm (Figure 1A). The main theory of this event explains that flow and pressure applied by the MV activate stretch receptors in upper airways, lungs and chest. As a result, the respiratory center coincides with the phase and frequency of the external stimulus (ventilator) producing a repetitive respiratory pattern [25]. In general, it is more likely to occur during prolonged mechanical lung insufflation, with low flow rates and high volumes. It has been reported that this entity is observed in 50% of patients with acute respiratory distress syndrome (ARDS) during the first 72 hours of MV [26]. When this phenomenon occurs periodically or synchronously with different patterns, it is called “entrainment,” and it can be present in awake sedated patients, even with neuromuscular blockade (NMB), taking place with major frequency when the programed TV and RR are close to the patient’s TV and RR. Its clinical impact will depend on the degree of mismatch between the mechanical cycle and the muscular effort. During RT, the patient’s inspiratory effort begins after the mandatory cycle, and generally, persists beyond it. It has been observed that the maximum expiratory flow is reduced significantly by the presence of inspiratory muscle activation at the beginning of expiration, preventing elastic recoil in the respiratory system.
RT has two principal characteristics: it occurs in a stable, repetitive pattern, and reflex-triggered ventilations differ minimally in timing, duration and magnitude of inspiratory effort. In order to detect and differentiate this entity, a prolonged expiratory pause has been proposed [27], to suppress the external stimulus and avoid diaphragm activation. To distinguish it from a DT, it is suggested to evaluate the pressure curve, where a negative deflection could mean the presence of a DT, but not a RT.
To characterize this entity, the existence of different phenotypes has been proposed depending on the moment of the ventilatory cycle in which they occur. This classification includes: early RT with early relaxation, early RT with delayed relaxation, mid-cycle RT, late RT, and RT with breath stacking. These variants may be associated with increased TV, increased mean airway pressure, increased end-inspiratory transpulmonary pressure, increased muscle pressure during expiration, persistent inspiratory muscle activity, decreased expiratory flow, and/or air trapping [28]. The same variants were also found in patients with ARDS [29].
To correct this asynchrony, the interruption and modification of the entrainment pattern is completely necessary. It is not suggested to increase sedation levels as treatment. Ventilation stacking can be prevented with the reduction of ventilator sensibility, but this action does not eradicates muscular effort unless TV and RR are changed. Although low TV and short inspiratory times recommended in protective ventilation may increase the risk of DT, it is noteworthy that, in ARDS Network study, patients who received TV of 8 ml/kg had fewer lapses of asynchrony than those who received 6 ml/kg, showing benefit by increasing TV, as long as there is no additional risk of superimposed lung injury [30].
Strategies like modification of TV, RF and the use of NMB have been described for the management of RT, although they only achieved a temporary resolution. He et al. [31] demonstrated that the reduction of the RR, without modifying other variants, decreased the percentage of RT by 30%. Rodriguez et al. [26] also identified the usefulness of RF reduction to solve TR in patients with ARDS. In view of these strategies to increase TV or decrease RF, it is important to monitor lung protection goals and CO2.
Auto Trigger
Auto-trigger can be described as the opposite of ineffective trigger. In this case, the MV triggers a ventilatory cycle without the presence of an inspiratory effort, mistakenly recognizing a variation in airway flow or pressure. In a recent review [2], auto-trigger is arbitrarily defined as one of the most important asynchronies, being considered in 45 studies of the mentioned work (73% of the studies analyzed).
Identification of this asynchrony should be suspected by displaying a total frequency higher than the one programmed on the MV with controlled modes, or a higher frequency without patient effort in assisted modes (Supplementary Figure 1E). This asynchrony can be caused by two circumstances: related to the MV or to the patient. Within the causes of the first term we can find air leaks, low sensitivity adjustments and circuit condensation. Patient-related causes include the transmission of intrathoracic pressure variations resulting from intense cardiac activity [32]. In the flow curve, specifically in the expiratory phase, constant and rhythmic oscillations or turbulence can be observed, which should make us suspect transmission of cardiac activity or circuit condensation. One option to confirm this singularity is to increase the sensitivity of the trigger and observe reduction in RR, secondary to the absence of effort or triggering in ventilatory cycles. Considering this, it is important to increase trigger sensitivity, correct leaks and remove existing circuit condensation [33].
Ineffective Trigger
This type of asynchrony is defined as inspiratory efforts that cannot be detected by the mechanical ventilator, and therefore, cannot start a ventilatory cycle, causing failure in the patient’s ventilatory demand. This asynchrony is only found in patients with spontaneous effort, so it cannot be detected in deeply sedated patients or those with NMB [34]. At the presence of an ineffective trigger, the patient’s RR will be higher than the total RR displayed by the ventilator. In a prospective observational study, Blanch et al. [20] evaluated the prevalence of asynchrony during different ventilatory modes, and noticed the high frequency of ineffective trigger, therefore, its early detection and correction is crucial.
Ineffective trigger is identified in the flow curve, during mid or end of expiration by an increase in inspiratory flow. Simultaneously, a pressure drop is displayed in the pressure curve (Supplementary Figure 1A). Most of time it is detected during expiration, however, it can also occur during inspiration [35]. This entity may be product of setting the trigger sensitivity too high or a long inspiratory time. Other causes include respiratory muscle weakness, decreased respiratory drive, inadequate programmed positive end-expiratory pressure (PEEP) and dynamic hyperinflation (auto-PEEP). All of this causes are related to the individual characteristics of each patient.
As a primary solution, sensitivity of the MV should be reduced. This action will help the patient to start the ventilatory cycle more easily. It is also necessary to reduce inspiratory time in different ventilatory modes and optimize infusion of sedative drugs or respiratory drive depressants. Decreasing dynamic hyperinflation by reducing pressure support (PS) levels and correctly titrating PEEP (to compensate auto-PEEP) are also options in patients with this problem [33].
Trigger Delay
The activation phase (or trigger) runs from the moment in which the patient initiates the effort until the opening of the inspiratory valve and the start of the flow supply. In modern MVs, response time is <100 ms, as long as trigger sensitivity is correctly stablished [36]. Trigger delay is defined as the presence of a discrepancy between the beginning of the patient’s effort and the time it takes for the MV to detect the mentioned effort [37]. This mainly occurs with inappropriate sensitivity setting (usually seen when sensitivity is too “hard,” making the MV triggering more difficult), causes related with the ventilator itself such as position of the flow/pressure sensor and high resistances produced by a heat and moisture exchanger. Problems related to the ventilator valves or endotracheal tube are also factors that can predispose to the appearance of this asynchrony.
Double Triggering: “Breath Stacking”
DT is classified as a flow asynchrony or insufficient assistance, but it is also classified as a trigger alteration. DT is composed of two continuous ventilatory efforts, both are initiated by the patient (unlike RT), with insufficient expiratory time between them, calculated at less than 50% average of the inspiratory time. Graphically, two stacked and continuous breaths are displayed in the pressure curve, calling this “breath stacking” [2] (Supplementary Figure 1F).
Due its clinical impact on mortality [20], it can be considered as a “major” asynchrony, therefore, adequate programming of protective ventilation and appropriate analgesia protocols are important. Programming a low TV predisposes to the appearance of this asynchrony. It must be considered that the volume of the stacked breaths can double the set TV in volume control-continuous mandatory ventilation with constant flow [38]. It is generally seen in patients with ARDS and protective ventilation, inadequate or low inspiratory flow, and high respiratory drive. Other causes may be related to a short inspiratory time, where cycling occurs before the Pmus peak, as well as the presence of other types of asynchrony such as premature cycling. It is worth mentioning that its appearance is more frequent in volume controlled mode [17,22,33].
Resolution of DT is achieved by optimized levels of sedation and analgesia, which may be increased if the patient requires strict protective ventilation. The use of NMB may also be considered. In those patients who, due to their clinical condition, do not require deep sedation, ventilatory spontaneous mode is suggested. Other options to consider may be: increase of TV, ventilatory support, inspiratory flow or time, or decrease of rise time. These interventions are intended to optimize ventilatory demand or “air hunger” [12,21].
FLOW ASYNCHRONIES
Insufficient Flow Asynchrony: “Air Hunger”
This asynchrony is based on the insufficient administration of gas flow, and, as a consequence, it is not possible to satisfy the patient's ventilatory demand. Consequently, it causes the activation and greater energy expenditure of inspiratory muscles. It can be displayed in volume-controlled modes, as a concavity in the pressure curve during the inspiratory phase (until peak pressure is reached), once the present inspiratory flow has been delivered. This phenomenon indicates patient’s inspiratory effort (Pmus) by air suction mode, which decreases the ventilatory pressure (Pvent), causing a “work shifting,” with the attempt to satisfy their own ventilatory demand.
This concavity will be directly proportional in magnitude to the inspiratory effort, and may generate deflection and pressure drop until triggering a second breathing, that is, a double trigger. It is called “air hunger” asynchrony for this reason [5,17]. Causes of this asynchrony are a low inspiratory flow or even a low TV, as in protective ventilation for ARDS. Other causes may be related to situations that generate a high respiratory drive such as agitation, pain and/or fever.
Its approach can be carried out by controlling anxiety states and adequate analgesia, increase in inspiratory flow and TV in volume-controlled mode, or by reducing the “rise time” in pressure-controlled modes. If the patient has criteria for spontaneous ventilation, switching to pressure support ventilation (PSV) and early extubation may also represent a solution, otherwise, adjust sedation to avoid inspiratory effort during protective ventilation for ARDS [17]. This entity can be considered as severe asynchrony due to the discomfort it generates, excessive inspiratory efforts, high trans pulmonary pressure gradients and potential lung injury or barotrauma (Supplementary Figure 1B and Figure 3) [5].
Flow Excess Asynchrony: “Overshooting”
It is a rare asynchrony compared to insufficient flow asynchrony because it is more likely to program a low flow than a high flow. It is mainly observed in volume-controlled mode, where inspiratory flow is excessive or higher than the patient’s demand, causing overcompensation (Figure 1B). It can also occur in pressure controlled modes, due to high programming of inspiratory pressure (iP) or PS, which generates excessive pressurization. Another option could be a shorter rise time.
Its identification is made more easily in pressure curve: a spike shape or peak will be appreciated on the first part of the inspiratory phase. Its clinical involvement may be minimal, causing only discomfort, however, in PSV mode it can generate early or premature cycling (due to excessively high flow after an inspiratory flow drop), thus shortening inspiratory phase and, consequently, generating an inadequate TV. An alternative to solve this clinical manifestation is to reduce inspiratory flow (in volume control mode) until it meets the patient’s demand or obtains an inspiration/expiration ratio close to 1:2, without causing air trapping. In pressure-controlled modes, decreasing PS or iP assistance, as well as increasing rise time, could represent viable solutions [5,32].
CYCLING ASSYNCHRONY
Cycling or expiratory asynchrony can be described from the relationship between neural inspiratory time and mechanical inspiratory time (MIT). NIT is defined as a breath that will be initiated and conclude by the patient and is in direct relation to respiratory drive. On the other hand, in MIT, the respiratory cycle will be initiated and concluded by the MV according to the programmed parameters, which will have a direct impact on the cycle, when the patient begins to present assisted or spontaneous breathing (sedation interruption, for example).
Within this classification, premature cycling and late cycling can be found. The first one is defined and caused by a programming the shortest MIT in relation to NIT. This situation stablish that MV finishes the inspiratory phase before the patient and, depending on NIT intensity or duration, may cause interruption of a peak expiratory flow and the start of a new respiratory cycle. This asynchrony can cause the presence of DT and generate greater lung injury due to "volutrauma" (Supplementary Figure 1C).
In late cycling, MIT is longer than NIT, thus, the MV will continue to deliver flow to the respiratory system at the same time that the patient has already ceased his inspiratory phase [39]. Consequently, during the end of the respiratory cycle, an increase in airway pressure and, in some cases, an increase in peak expiratory flow may be observed. The persistence of this entity can trigger a lung injury due to “barotrauma” (Supplementary Figure 1D).
In those patients where the cause of the mechanical respiratory assistance has been resolved, and they are stable from the respiratory and hemodynamic point of view, it is suggested to use a spontaneous ventilation modality in order to avoid the appearance of asynchrony. On the other hand, patients who require moderate or conscious sedation (Richmond Agitation-Sedation Scale -2 or -3) without the need for a neuromuscular blocker (assisted modality), could have variability between both neural and the mechanical times, giving rise to asynchrony. It is important to prioritize NIT over MIT taking into account the RR and inspiratory time. Likewise, observe "coupling" once the parameters have been adjusted, avoiding a low or high TV, CO2 retention or the appearance of a new asynchrony.
EXPIRATORY MUSCLE RELAXATION-INDUCED TRIGGERING
Recently, a reported case [4] described a patient with contraction of the abdominal muscles during the expiratory phase and, before the cessation of this activity, the start of a new respiratory cycle (initiated by the ventilator) without initial activity of the diaphragm, which is later activated. Under normal physiological conditions, expiration is a passive process, secondary to the retraction of the lung tissue and the cessation of the concentric contraction of the diaphragm. However, this situation is not always observed: sustained and maximal contraction of the expiratory muscles, for example, can bring the lung down to its residual volume, shedding the expiratory reserve volume (ERV). As a consequence, to return to the normal functional residual capacity, it’s necessary to reincorporate ERV by expanding the thoracic cage to equalize pressures with the lung, generating a negative pressure that, if intense enough, will trigger a new cycle in the MV, leading to asynchrony.
The diaphragm action, as described, will be activated lately, showing a mismatch between the start of the mechanical ventilatory time and the start of the neural inspiratory cycle. Possibly, the diaphragm will be activated by a reflex mechanism initiated after the "rebound effect" that returns the system to its equilibrium point. Thus, a double respiratory cycle is observed (Supplementary Figure 1G).
Despite not having specifically designed studies to address this new asynchrony, we will test some options to prevent its deleterious effects. Considering that asynchrony is a consequence of muscular activity, optimize level of sedation, analgesia and, if necessary, neuromuscular blockers could be a possible solution. The use of bronchodilators should also be considered to reduce airflow resistance and prevent contraction of the abdominal muscles. Finally, there would be an option based on MV parameter settings. However, this last option is complex to implement since mechanical times will be stable and neural times will be variable.
MONITORING
Visual Inspection
Visual inspection of flow/time and pressure/time curves is a traditionally accepted and reliable method for identifying asynchronies, but requires specific skills and experience for correct interpretation. Colombo et al. [40] evaluated the ability of ICU physicians to detect dyssynchrony during PSV, by inspecting flow and pressure waveforms, and to determine the impact of physician experience on the ability to recognize asynchrony; found that the ability to correctly recognize asynchronies is generally quite low and is only moderately influenced by clinical experience. ICU physicians can detect less than a third of dyssynchronies, which is, however, significantly higher than the 16% detection rate shown by ICU residents [40].
Likewise, Ramirez et al. [41] carried out an observational study that included 366 health professionals (physicians, respiratory therapists and nurses) in 17 urban ICUs. These professionals were shown three videos with different asynchronies and were asked to identify them. A significant difference was found between professionals who had training in MV versus those who did not. Thus, among the trained professionals, 81% managed to correctly identify the three asynchronies versus 19% in the untrained (P<0.001). In the same way, professionals who had training increased the chance of identifying two or more asynchronies adequately by almost four times (odds ratio, 3.67; 95% confidence interval, 1.93–6.96; P<0.001). It is important to note that in this study neither years of experience nor profession were associated with the ability of professionals to correctly detect asynchrony [41].
Ultrasound
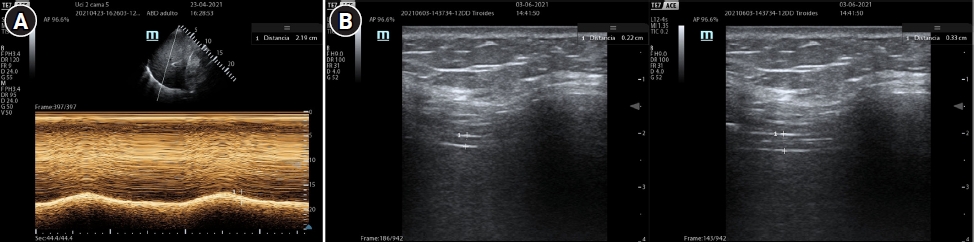
Diaphragm ultrasound (Figure 4) could be a reasonable alternative to detect some types of asynchrony. It has the advantage of being a non-invasive method, the learning curve is achieved quickly and it is harmless to the patient. Direct observation of the diaphragmatic movement, both in its displacement and in its thickening fraction, allow to detect the patient's inspiratory effort and its magnitude. Soilemezi et al [42], reported a case series where, through ultrasonography, along with esophageal pressure, they detected cases of DT, RT and ineffective trigger. This simple approach is not yet standardized and requires synchronization of the ventilator curves with the ultrasound signal, so its use still requires investigation.
Esophageal Pressure
One of the main challenges in monitoring and detecting asynchrony is being able to estimate the activity of the respiratory muscles, a task that is not always easy and could go unnoticed, even by experts. This difficulty justifies the use of an esophageal signal (Pes), through the placement of a balloon in the esophagus connected to a transducer to monitor pressure changes and be able to estimate the beginning, the end and the magnitude of muscular effort. Pes recording can help detect asynchrony by comparing the temporal occurrence of changes in Pes with changes in Paw and waveforms on the flow-time curve. The use of Pes allows to detect the inspiratory effort simultaneously with the Paw curves and to monitor the synchronicity between both curves to match the patient's inspiratory effort with each mechanical cycle (an IE can be identified as a negative deflection of Pes that is not followed by a ventilation cycle, for instance). The main disadvantages of this technique are its invasiveness and not being available in all ICUs [43].
CONCLUSION
MV is a life-saving therapy. Its correct implementation requires a learning curve and theoretical concepts to turn it into a safe tool. Even so, it is not exempt from adverse effects that can be injurious to the lung and prolong the application time of the therapy and ICU length of stay. In this sense, the patient-ventilator interaction represents a challenge for intensivists, respiratory therapists and other health professionals. When an adequate balance between patient´s needs and the assistance offered by the ventilator is not achieved, asynchrony begins to appear frequently. That is why it is mandatory to deepen the understanding of the principles of physiology and the mechanics of the respiratory system, to understand and identify the causes that generate this imbalance, guaranteeing a correct interpretation of the different entities and optimizing the clinical practices and outcomes
KEY MESSAGES
▪ Asynchronies during mechanical ventilation have shown association with worse outcomes.
▪ Early detection and treatment could improve patient survival.
▪ There are different classifications of asynchrony.
▪ A distinction based on phase variables helps to better understand its causes and early detection.
▪ Complementary methods, such as ultrasonography, esophageal pressure or visual inspection, collaborate and reinforce the detection, diagnosis and treatment of these entities.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: SNS, PVSB, JBPM, AGG, AG. Data curation: SNS, PVSB, JBPM, AG. Formal analysis: SNS, JBPM, AG. Methodology: all authors. Project administration: SNS, AG. Visualization: AG. Writing–original draft: all authors. Writing–review & editing: PVSB, JBPM, AGG, AG.
Acknowledgments
None.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2022.01158.
Supplementary Figure 1.
Different types of asynchronies. (A) Ineffective trigger. (B) Insufficient flow. (C) Premature cycling. (D) Delay cycling. (E) AutoTrigger. (F) Double trigger. (G) Expiratory muscle relaxation-induced triggering.
acc-2022-01158-suppl.pdf
Figure 1.(A) Reverse triggering. (B) Overshooting.

Figure 2.Classification of asynchronies according to phrase variables.
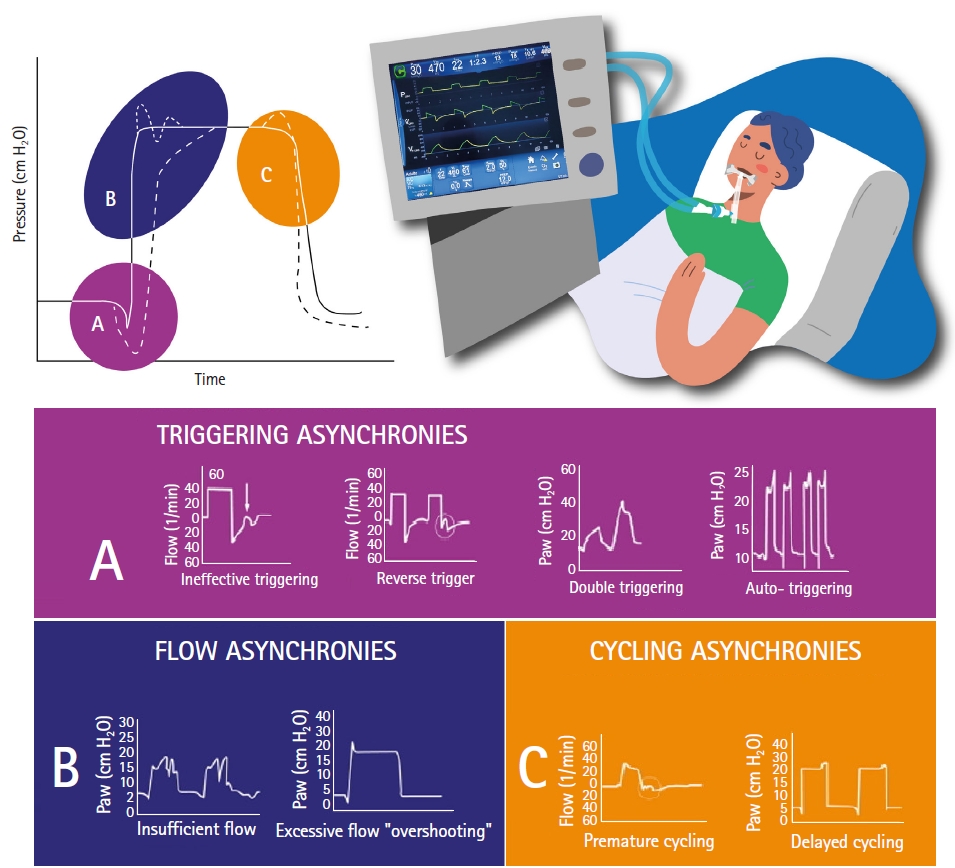
Figure 3.Different types of ventilation-induced lung injury due to asynchronies.
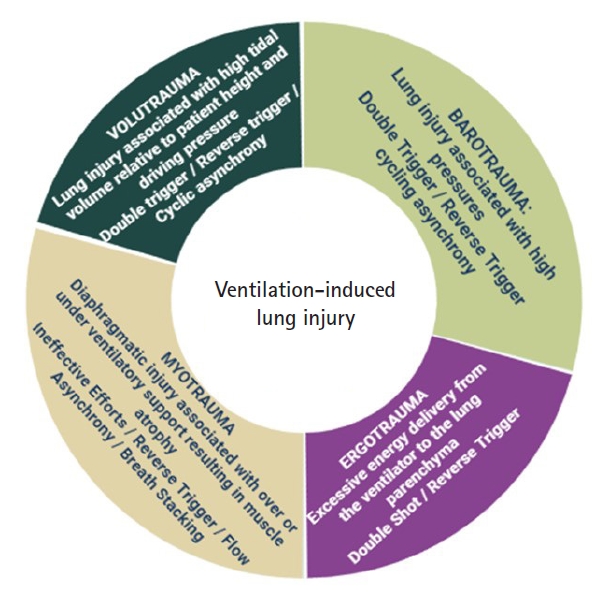
Figure 4.Ultrasound monitoring of the diaphragmatic function. (A) Diaphragmatic excursion. (B) Diaphragmatic thickening fraction.
References
- 1. Wunsch H, Linde-zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010;38:1947-53.ArticlePubMed
- 2. Bruni A, Garofalo E, Pelaia C, Messina A, Cammarota G, Murabito P, et al. Patient-ventilator asynchrony in adult critically ill patients. Minerva Anestesiol 2019;85:676-88.ArticlePubMed
- 3. Magrans R, Ferreira F, Sarlabous L, López-Aguilar J, Gomà G, Fernandez-Gonzalo S, et al. The effect of clusters of double triggering and ineffective efforts in critically ill patients. Crit Care Med 2022;50:e619-29.ArticlePubMed
- 4. Jonkman AH, Holleboom MC, de Vries HJ, Vriends M, Tuinman PR, Heunks LM. Expiratory muscle relaxation-induced ventilator triggering: a novel patient-ventilator dyssynchrony. Chest 2022;161:e337-41.ArticlePubMedPMC
- 5. Oto B, Annesi J, Foley RJ. Patient-ventilator dyssynchrony in the intensive care unit: a practical approach to diagnosis and management. Anaesth Intensive Care 2021;49:86-97.ArticlePubMedPDF
- 6. Barwing J, Pedroni C, Olgemöller U, Quintel M, Moerer O. Electrical activity of the diaphragm (EAdi) as a monitoring parameter in difficult weaning from respirator: a pilot study. Crit Care 2013;17:R182. ArticlePubMedPMC
- 7. Hickey SM, Giwa AO. Mechanical ventilation [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 Jan 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539742/.
- 8. Chen S, Li Y, Zheng Z, Luo Q, Chen R. The analysis of components that lead to increased work of breathing in chronic obstructive pulmonary disease patients. J Thorac Dis 2016;8:2212-8.ArticlePubMedPMC
- 9. Cabello B, Mancebo J. Work of breathing. Intensive Care Med 2006;32:1311-4.ArticlePubMedPDF
- 10. French CJ. Work of breathing measurement in the critically ill patient. Anaesth Intensive Care 1999;27:561-73.ArticlePubMedPDF
- 11. Parthasarathy S, Jubran A, Tobin MJ. Assessment of neural inspiratory time in ventilator-supported patients. Am J Respir Crit Care Med 2000;162(2 Pt 1):546-52.ArticlePubMed
- 12. Shah VH, Samanta A, Ray S. Patient-ventilator asynchrony: etiology and solutions. Indian J Clin Pract 2021;31:714-24.
- 13. Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 2020;46:606-18.ArticlePubMedPMCPDF
- 14. Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients: pathophysiology and clinical implications. Am J Respir Crit Care Med 2020;201:20-32.ArticlePubMed
- 15. Jonkman AH, de Vries HJ, Heunks LM. Physiology of the respiratory drive in ICU patients: implications for diagnosis and treatment. Crit Care 2020;24:104. ArticlePubMedPMCPDF
- 16. Telias I, Spadaro S. Techniques to monitor respiratory drive and inspiratory effort. Curr Opin Crit Care 2020;26:3-10.ArticlePubMed
- 17. Pham T, Telias I, Piraino T, Yoshida T, Brochard LJ. Asynchrony consequences and management. Crit Care Clin 2018;34:325-41.ArticlePubMed
- 18. Varon J, Fromm R, Rodarte J, Reinoso M. Prevalence of patient ventilator asynchrony in critically ill patients. Chest 1994;106(2 Suppl):141S-144S.Article
- 19. Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006;32:1515-22.ArticlePubMedPDF
- 20. Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Luján M, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 2015;41:633-41.ArticlePubMedPDF
- 21. Kyo M, Shimatani T, Hosokawa K, Taito S, Kataoka Y, Ohshimo S, et al. Patient-ventilator asynchrony, impact on clinical outcomes and effectiveness of interventions: a systematic review and meta-analysis. J Intensive Care 2021;9:50. ArticlePubMedPMCPDF
- 22. Mireles-Cabodevila E, Siuba MT, Chatburn RL. A taxonomy for patient-ventilator interactions and a method to read ventilator waveforms. Respir Care 2022;67:129-48.ArticlePubMed
- 23. Esperanza JA, Sarlabous L, de Haro C, Magrans R, Lopez-Aguilar J, Blanch L. Monitoring asynchrony during invasive mechanical ventilation. Respir Care 2020;65:847-69.ArticlePubMed
- 24. Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31.ArticlePubMed
- 25. de Vries HJ, Jonkman AH, Tuinman PR, Girbes AR, Heunks LM. Respiratory entrainment and reverse triggering in a mechanically ventilated patient. Ann Am Thorac Soc 2019;16:499-505.ArticlePubMed
- 26. Rodriguez PO, Tiribelli N, Fredes S, Gogniat E, Plotnikow G, Fernandez Ceballos I, et al. Prevalence of reverse triggering in early ARDS: results from a multicenter observational study. Chest 2021;159:186-95.ArticlePubMed
- 27. Dianti J, Bertoni M, Goligher EC. Monitoring patient-ventilator interaction by an end-expiratory occlusion maneuver. Intensive Care Med 2020;46:2338-41.ArticlePubMedPMCPDF
- 28. Baedorf Kassis E, Su HK, Graham AR, Novack V, Loring SH, Talmor DS. Reverse trigger phenotypes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2021;203:67-77.ArticlePubMedPMC
- 29. Lin Z, Zhou J, Lin X, Wang Y, Zheng H, Huang W, et al. Reverse trigger in ventilated non-ards patients: a phenomenon can not be ignored! Front Physiol 2021;12:670172. ArticlePubMedPMC
- 30. Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8.ArticlePubMed
- 31. He X, Luo XY, Chen GQ, Zhou JX. Detection of reverse triggering in a 55-year-old man under deep sedation and controlled mechanical ventilation. J Thorac Dis 2018;10:E682-5.ArticlePubMedPMC
- 32. Imanaka H, Nishimura M, Takeuchi M, Kimball WR, Yahagi N, Kumon K. Autotriggering caused by cardiogenic oscillation during flow-triggered mechanical ventilation. Crit Care Med 2000;28:402-7.ArticlePubMed
- 33. Holanda MA, Vasconcelos RD, Ferreira JC, Pinheiro BV. Patient-ventilator asynchrony. J Bras Pneumol 2018;44:321-33.ArticlePubMedPMC
- 34. Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med 2006;32:34-47.ArticlePubMedPDF
- 35. Blanch L, Sales B, Montanya J, Lucangelo U, Garcia-Esquirol O, Villagra A, et al. Validation of the Better Care® system to detect ineffective efforts during expiration in mechanically ventilated patients: a pilot study. Intensive Care Med 2012;38:772-80.ArticlePubMedPDF
- 36. Sassoon CS. Triggering of the ventilator in patient-ventilator interactions. Respir Care 2011;56:39-51.ArticlePubMed
- 37. Mirabella L, Cinnella G, Costa R, Cortegiani A, Tullo L, Rauseo M, et al. Patient-ventilator asynchronies: clinical implications and practical solutions. Respir Care 2020;65:1751-66.ArticlePubMed
- 38. de Haro C, López-Aguilar J, Magrans R, Montanya J, Fernández-Gonzalo S, Turon M, et al. Double cycling during mechanical ventilation: frequency, mechanisms, and physiologic implications. Crit Care Med 2018;46:1385-92.ArticlePubMed
- 39. Holanda MA, Vasconcelos R, Ferreira JC, Pinheiro BV. Patient-ventilator asynchrony. J Bras Pneumol 2018;44:321-33.ArticlePubMedPMC
- 40. Colombo D, Cammarota G, Alemani M, Carenzo L, Barra FL, Vaschetto R, et al. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med 2011;39:2452-7.ArticlePubMed
- 41. Ramirez II, Arellano DH, Adasme RS, Landeros JM, Salinas FA, Vargas AG, et al. Ability of ICU health-care professionals to identify patient-ventilator asynchrony using Waveform analysis. Respir Care 2017;62:144-9.ArticlePubMed
- 42. Soilemezi E, Vasileiou M, Spyridonidou C, Tsagourias M, Matamis D. Understanding patient-ventilator asynchrony using diaphragmatic ultrasonography. Am J Respir Crit Care Med 2019;200:e27-8.ArticlePubMed
- 43. Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016;42:1360-73.ArticlePubMedPDF
Citations
Citations to this article as recorded by

- Patient Self-Inflicted Lung Injury—A Narrative Review of Pathophysiology, Early Recognition, and Management Options
Peter Sklienka, Michal Frelich, Filip Burša
Journal of Personalized Medicine.2023; 13(4): 593. CrossRef - Actualización sobre sedoanalgesia en paciente bajo ventilación mecánica
Onan Emanuel Gregorio
Revista de Postgrados de Medicina.2022; 1(1): 27. CrossRef
 , Patrick Valentino Sepúlveda Barisich2
, Patrick Valentino Sepúlveda Barisich2 , José Benito Parra Maldonado3
, José Benito Parra Maldonado3 , Romina Belén Lumini4
, Romina Belén Lumini4 , Alberto Gómez-González3
, Alberto Gómez-González3 , Adrián Gallardo5
, Adrián Gallardo5







 KSCCM
KSCCM



 PubReader
PubReader ePub Link
ePub Link Cite
Cite