Dear Editor:
Mortality due to sepsis has decreased with dissemination of the sepsis bundle. To successfully manage sepsis, it is essential to diagnose the source of infection, administer antibiotics, surgically treat if needed, and concurrently resuscitate the patient as soon as possible [1]. However, even when a patient is adequately managed, treatment can fail if the culprit pathogen cannot be identified. Such cases can require extensive analysis including rare pathogens. Invasive fungal disease (IFD) is suspected as a source of sepsis in immunocompromised hosts, and conventional risk factors are well established. Neutropenia, hematopoietic stem cell or solid organ transplantation, high-dose corticosteroid use, and immunosuppressant use are proven risk factors [1]. However, there have been cases of suspected IFD in patients with no conventional risk factors. Here, we present an interesting case of community-acquired pneumonia and septic shock in which invasive pulmonary aspergillosis was considered as the primary pathogenesis. The patient was anonymized, and the Institutional Review Board of Soonchunhyang University Bucheon Hospital approved this study (No. 2023-02-018). Because of the retrospective review of a discharged patient, the need for informed consent was waived.
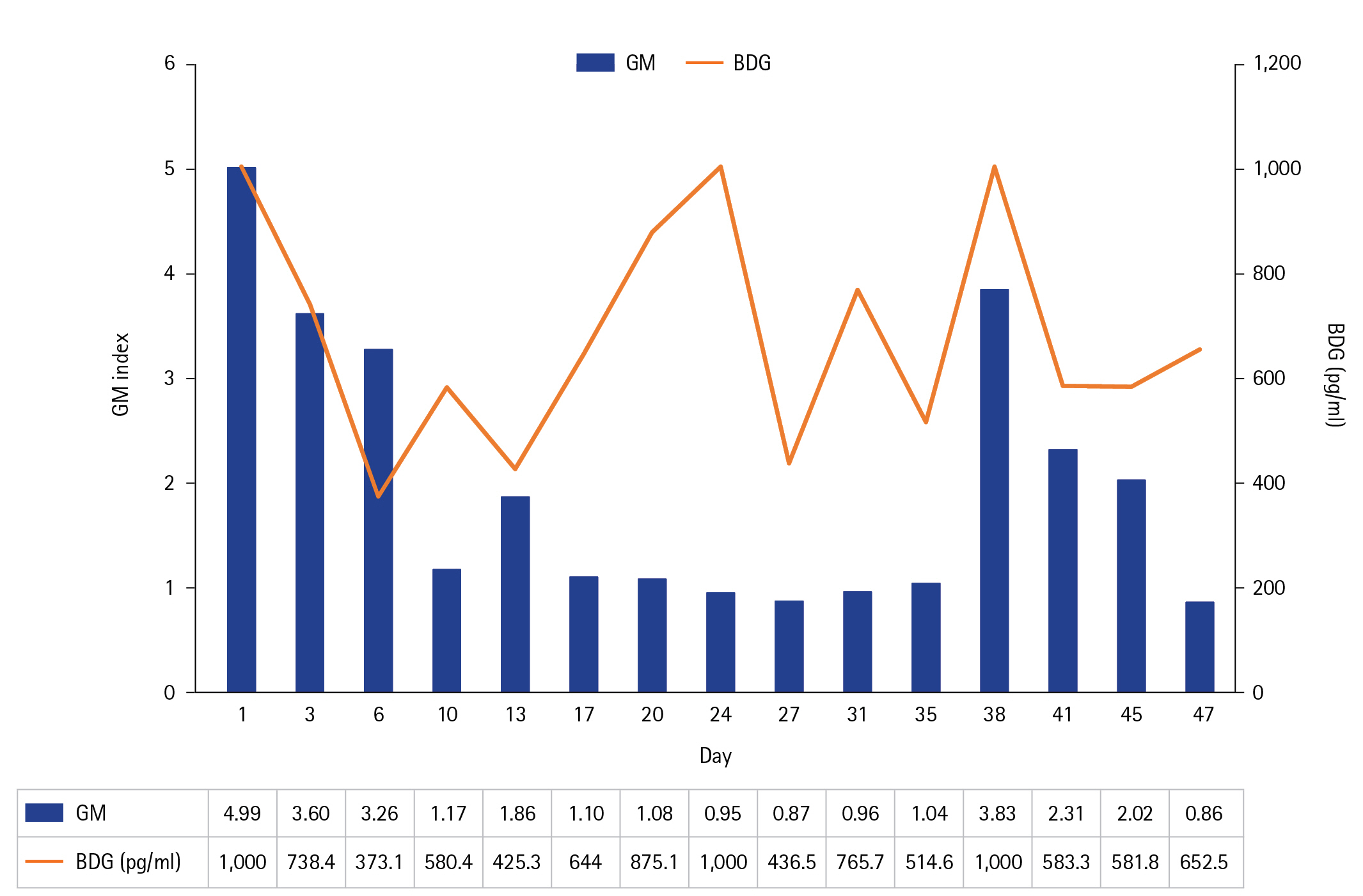
An 85-year-old man with a history of carotid artery stenosis, hyperlipidemia, chronic kidney disease, and dementia was admitted to the intensive care unit (ICU) because of sputum produced 1 day prior and worsening dyspnea on the visit day. Upon admission, the patient was drowsy, with blood pressure 69/37 mm Hg, heart rate 75 beats/min, respiratory rate 22 beats/min, body temperature 36.9 °C, and pulse oxygen saturation 60% at room air. Coarse breath sounds with crackles were heard in both lung fields. Concurrent with mechanical ventilation, adequate intravenous fluid and norepinephrine were administered. The initial serum lactate level was elevated to 2.5 mmol/L. Chest radiograph showed increased opacities mainly in the right lower lung field (Figure 1A). Chest computed tomography revealed multifocal nodules with surrounding ground-glass opacities (GGOs), the “halo sign” in underlying emphysematous lungs, and extensive consolidation with GGO in both lower lobes (Figure 2A and B). The initial white blood cell and absolute neutrophil counts were 3,110/μl and 2,430/μl,, which increased to 8,430/μl and 6,830/μl on the second day, respectively. Non-specific erythematous bronchial mucosa with a large amount of thick, purulent sputum was observed on bronchoscopy, and bronchial lavage and culture were performed. Serum high-sensitivity C-reactive protein (hs-CRP) and procalcitonin levels were 16.47 mg/dl and 20.9 ng/ml, respectively. The broad-spectrum antibacterial agent cefepime was infused immediately. During the first 5 days, the blood platelet count decreased markedly from 103,000 to 65,000 /µl; serum creatinine level increased from 1.7 to 2.0 mg/dl; and serum hs-CRP and procalcitonin levels increased to 18.07 mg/dl and 55.0 ng/ml, respectively. The initial bronchoscopic culture and respiratory virus multiplex tests showed negative results, except the galactomannan (GM) antigen level from bronchoscopic washing was elevated at 1.5. The serum GM antigen level was also elevated at 4.99 (Figure 3). Blood beta-D-glucan (BDG) level peaked at greater than 1,000.0 pg/ml. With suspicion of invasive aspergillosis, oral voriconazole was added on the third day of admission. The patient was not at high risk for multidrug-resistant organism infection related to recent exposure to antimicrobial drugs or hospital admission and was not at high risk for opportunistic infection due to immunosuppression. Ten days later, given that the hs-CRP level was still high at 16.06 mg/dl and the follow-up chest CT images revealed aggravation of nodular consolidations, oral voriconazole was replaced with intravenous liposomal Amphotericin B (Figure 2C). The hs-CRP level finally improved to 3.80 mg/dl 1 week after start of intravenous liposomal Amphotericin B. Finally, Aspergillus fumigatus was cultured on the 33rd day from the endotracheal aspirate sample collected on the 26th day of intensive care. The patient was weaned from the mechanical ventilator on the 40th day of ICU admission in the tracheostomy state and discharged from the ICU after 45 days of intensive care. The follow-up radiograph and CT images showed partial improvement of nodular consolidations (Figure 1B and 2D).
IFD may have a poor prognosis without a timely diagnosis, especially in non-neutropenic patients [2]. Prohaska et al. [3] reported a case of fatal acute respiratory distress syndrome with undiagnosed IFD, in which Mucor and Aspergillus mycelium were identified at autopsy. The patient had no conventional risk factor and died with an enigmatic course despite full coverage of the identified bacteria.
The diagnostic method of choice for proven invasive aspergillosis is biopsy. However, transcutaneous lung biopsy is associated with risk of iatrogenic pneumothorax and air embolism, especially in patients on mechanical ventilators. More noninvasive diagnostic tools are needed for IFD, especially for critically ill patients. In the latest statement for IFD in 2021, probable invasive aspergillosis was defined as either the presence of Aspergillus species or GM positivity in the serum and/or bronchoalveolar fluid, plus a supporting radiological finding and a supporting host factor. Among the host factors, chronic respiratory airway abnormality and severe viral pneumonia were included [4]. Some other risk factors for IFD have been proposed, including diabetes mellitus, alcoholism, malnutrition, and critical illness [2].
At first, our patient seemed to have no relevant risk factor for invasive aspergillosis, except slight emphysematous change in both lungs on chest CT. However, his serum and bronchial washing GM were positive, and the serum level of BDG [5], another fungal marker, was increased. Further, the radiologic findings were consistent with invasive aspergillosis, e.g., the “halo sign,” contrary to most non-neutropenic patients showing non-specific results [2]. Finally, we diagnosed the patient with probable invasive aspergillosis and prescribed antifungal treatment.
In critically ill patients who are unresponsive despite proper management, IFD, such as invasive aspergillosis, should be suspected because of its high mortality, even in patients without non-conventional risk factors. It is helpful to assess serum invasive fungal markers such as GM and BDG and nodular consolidations with surrounding GGO on CT images, instead of obtaining a more invasive lung biopsy. Non-conventional risk factors and non-invasive diagnosis of IFD should be investigated further and are considered in our current practice.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2022R1G1A1009464).
-
AUTHOR CONTRIBUTIONS
Conceptualization: ARB. Data curation: KES, ARB. Funding acquisition: ARB. Methodology: all authors. Project administration: ARB. Visualization: KES, ARB. Writing–original draft: KES, ARB. Writing–review & editing: all authors.
Acknowledgments
None.
Figure 1.(A) The initial chest radiograph shows increased opacities, mainly in the right lower lung field. (B) The last chest radiograph on the 74th day of admission reveals decreased opacities compared to (A).

Figure 2.(A) The coronal view of initial chest computed tomography (CT) shows bilateral multifocal nodules with surrounding ground-glass opacities (GGOs), the “Halo sign” (black arrows), and centrilobular emphysema (white arrowheads) in both lungs. (B) Axial images of the same CT scan reveal multiple nodules with surrounding GGO (black arrows in the above row) in both upper lobes and extensive consolidation with GGO (black arrows in the bottom row) in both lower lobes. (C) The follow-up axial CT images show aggravated nodular consolidations in the upper and lower lobes on the 13th day of admission. (D) The second follow-up axial chest CT images show partial improvement of nodular consolidations in the whole lung fields on the 45th day of admission.
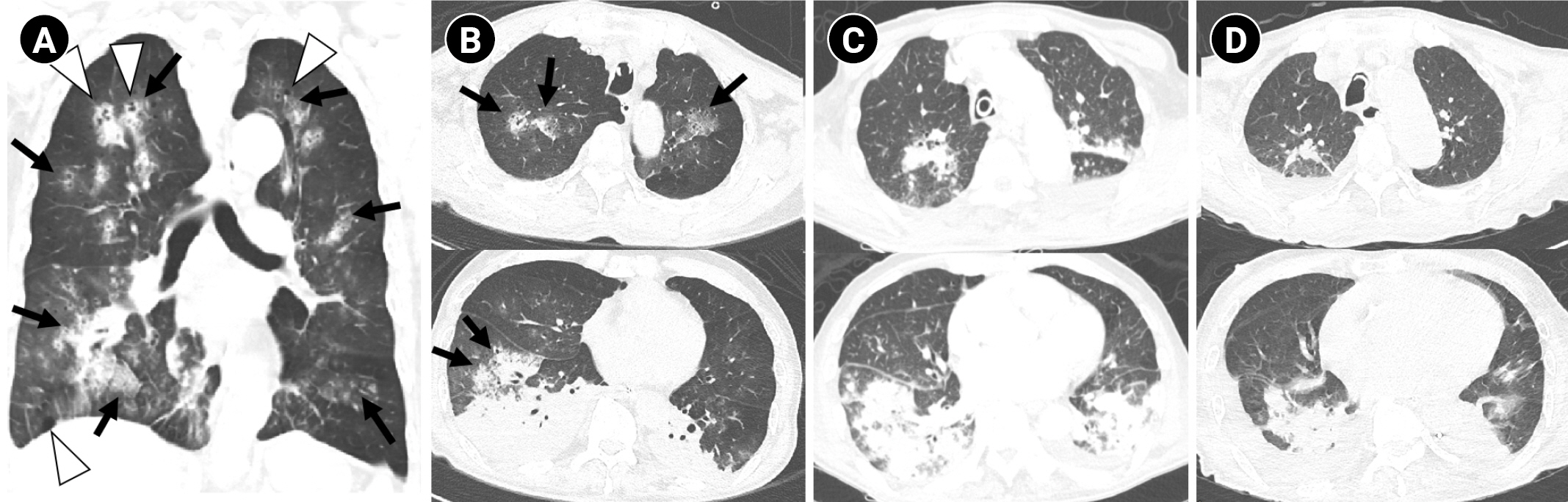
Figure 3.Serum invasive fungal markers of galactomannan antigen (GM) and beta-D-glucan (BDG) levels were initially high but decreased upon treatment, with a persistent and intractable disease course.
References
- 1. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021;49:e1063-143.PubMed
- 2. Bassetti M, Righi E, De Pascale G, De Gaudio R, Giarratano A, Mazzei T, et al. How to manage aspergillosis in non-neutropenic intensive care unit patients. Crit Care 2014;18:458. ArticlePubMedPMCPDF
- 3. Prohaska S, Henn P, Wenz S, Frauenfeld L, Rosenberger P, Haeberle HA. A case report of fatal disseminated fungal sepsis in a patient with ARDS and extracorporeal membrane oxygenation. BMC Anesthesiol 2020;20:107. ArticlePubMedPMCPDF
- 4. Bassetti M, Azoulay E, Kullberg BJ, Ruhnke M, Shoham S, Vazquez J, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis 2021;72(Suppl 2):S121-7.ArticlePubMedPDF
- 5. Acosta J, Catalan M, del Palacio-Pérez-Medel A, Montejo JC, De-La-Cruz-Bértolo J, Moragues MD, et al. Prospective study in critically ill non-neutropenic patients: diagnostic potential of (1,3)-β-D-glucan assay and circulating galactomannan for the diagnosis of invasive fungal disease. Eur J Clin Microbiol Infect Dis 2012;31:721-31.ArticlePubMedPDF
Citations
Citations to this article as recorded by

 , Shinhee Park2
, Shinhee Park2 , Ae-Rin Baek2
, Ae-Rin Baek2







 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite




