Abstract
-
Background
- Coronary atherosclerosis is the leading cause of coronary artery disease. Several investigations have indicated that tear-sensitive plaques contain macrophages and T cells. Neopterin is an essential cellular immune response biomarker. The main goal of this study was to see if there were any changes in biomarkers like unconjugated pteridines, neopterin, and biopterin, as well as kynurenine pathway enzymes like indoleamine 2,3-dioxygenase (IDO), which catalyzes the rate-limiting step in tryptophan degradation, in patients with the acute coronary syndrome (ACS) caused by angiographic atherosclerosis.
-
Methods
- High-performance liquid chromatography was used to determine the amounts of neopterin, biopterin, and creatinine in urine samples, as well as tryptophan and kynurenine in serum samples. The enzyme-linked immunosorbent assay was used to assess the amounts of neopterin in serum samples. The measured parameters were evaluated between ACS patients and controls.
-
Results
- The measured levels of neopterin, biopterin and the kynurenine to tryptophan ratio reflecting IDO activity, and the specifically known biomarkers such as cardiac troponin, creatine kinase, myoglobin, and natriuretic peptides are statistically higher in ACS patients compared to control subjects. On the other hand, the measured parameters are inadequate to classify the conventional kinds of ACS, ST-elevation- and non-ST-elevation- myocardial infarction.
-
Conclusions
- The study found that determining and using neopterin and IDO parameters as biomarkers in individuals with the ACS can support traditional biomarkers. However, it can be concluded that evaluating pteridine biomarkers solely have no privilege to clinical findings in ACS diagnosis and classification.
-
Keywords: acute coronary syndrome; indoleamine 2,3-dioxygenase; kynurenine; neopterin; ST elevation myocardial infarction; troponin; tryptophan
INTRODUCTION
The term acute coronary syndrome (ACS) refers to sudden myocardial ischemia or infarction induced by coronary circulation abnormalities. Atherosclerotic plaque rupture with thrombotic occlusion of a coronary artery, plaque degradation with endothelial dysfunction, microembolization, coronary vasospasm, toxicity from excess catecholamines, and coronary artery dissection are all symptoms of ACS. At first, ACS was characterized solely by symptoms and clinical signs. Simultaneously, electrocardiograms (ECGs), coronary angiography, more sensitive laboratory testing, and numerous new imaging modalities were added to the diagnosis [1,2]. The three conventional kinds of ACS are non-ST-elevation myocardial infarction (NSTEMI), ST-elevation myocardial infarction (STEMI), and unstable angina [3].
Comprehensive study over the last 20 years has produced compelling evidence that inflammation plays a significant role in the genesis and advancement of atherosclerosis. C-reactive protein (CRP), amyloid A, interleukin-6, matrix metalloproteases, and other known biomarkers of inflammation have been shown to have a significant role in the creation and evolution of atherosclerotic plaques [4]. In recent years, numerous epidemiological studies have indicated that serum levels of another well-known inflammatory biomarker, neopterin, increased significantly in people with coronary artery disease (CAD) and ACS. [4].
Neopterin is a pteridine molecule produced as a by-product of the guanosine triphosphate-biopterin pathway. It is produced by macrophages, which release it when activated. Because macrophages are actively implicated in inflammatory pathways, neopterin is regarded as a measure of macrophage activity and, thus, an inflammatory biomarker. Similarly, measures of neopterin levels in biological fluids are commonly employed in the clinical evaluation of patients to assess the course of viral infections, renal transplant rejection, severe systemic inflammatory illnesses, nephritic syndrome, and other autoimmune diseases [4,5]. Considering the relatively new idea that inflammation is important in the pathogenesis of atherosclerosis, it is exciting to investigate neopterin's active participation within vulnerable plaque for ACSs [4]. Tetrahydrobiopterin (BH4), an unconjugated pteridine compound, can be found in several forms in biological fluids, including dihydrobiopterin and biopterin, which are also excreted in urine. BH4 is a catalyst in several enzymatic reactions, including nitric oxide synthase, alkyl-glycerol monooxygenase, and aromatic amino acid hydroxylases, including tryptophan hydroxylase [6]. BH4 insufficiency can cause hyperphenylalaninaemia and decreased synthesis of neurotransmitters such as dopamine, norepinephrine, epinephrine, and serotonin [7,8].
The kynurenine (Kyn) route is the principal metabolic pathway for degrading the vital amino acid tryptophan (Trp). The Kyn pathway's rate-limiting enzymes are tryptophan 2,3-dioxygenase (TDO2) and indoleamine 2,3-dioxygenase 1 (IDO1) [9]. According to recent studies, the Kyn pathway is linked to atherosclerosis, showing that Trp catabolism in the arterial wall via this route is crucial for maintaining vascular immune homeostasis. Kyn to Trp ratio (Kyn/Trp) expresses IDO enzyme activity. In this circumstance, a high Kyn/Trp is found in patients with CAD, such as unstable angina, acute myocardial infarction, and sudden cardiac arrest [10-12].
The major aim of the present study is to evaluate the changes in neopterin, biopterin, and Kyn pathways in patients with ACS and its sub-groups STEMI and NSTEMI by comparing them with controls. In order to find out if these parameters can be included in the clinical evaluation of ACS; the measured parameters were also compared and correlated with current biomarkers such as troponin, creatine kinase, natriuretic peptide, CRP, and low-density lipoproteins (LDLs).
MATERIALS AND METHODS
Study Groups and Sampling
A power analysis was conducted to determine the minimum sample size required to test the study hypothesis. The required sample size to achieve 90% power for detecting medium effect was 30, with a confidence level of 95. The study population was comprised of 70 patients who were present at the Hospital's Emergency Medicine Department. A summary of the study groups is given in Figure 1. The study included patients older than 18 years and 50 patients (14 females, 36 males) diagnosed with ACS. Blood and urine samples were taken from the patients (n=50) before coronary angiography. ACS patients based on their ECG were sub-grouped, such as STEMI with new acute ST-elevation on the ECG and NSTEMI with ST-segment depression, T-wave changes, or no ECG abnormalities [13]. According to ECG, the ACS patients were subdivided into the STEMI group (n=30) and NSTEMI (n=20). Patients were followed for 6 months; 6 underwent balloon angioplasty, 12 underwent coronary bypass surgery, 26 had stents, and 6 used the medicine.
The control group (8 females, 12 males) was formed from relatively healthy individuals with no inflammation or infectious disease and randomly selected from patients who applied to the emergency department. The patients were transferred from the emergency clinic to cardiology clinic for acute myocardial infarction care.
Ethical Approval
The University's Local Ethical Committee of Scientific Research and Clinical Trials authorized the study's methodologies (No. 2017/01, No. 14). The study was carried out following the Helsinki Declaration. All participants were informed of all trial details, and their written consent was taken.
Measurements
High-performance liquid chromatography (HPLC; HP Agilent 1100) was used to determine the levels of neopterin, biopterin, and creatinine in urine samples. Reversed-phase chromatography was utilized at a flow rate of 1 ml per minute with a mobile phase of 15 mM phosphate buffer (pH 7) containing 2.5% (v/v) methanol. A fluorescence detector at 353 nm excitation and 438 nm emission wavelengths was used to measure neopterin and biopterin levels. In contrast, a UV detector at 235 nm (HP Agilent 1100) was used to measure urinary creatinine levels. The concentrations of neopterin and biopterin in the urine were calculated as µmol/mol creatinine.
All participants had drawn peripheral venous blood samples (3–5 mL). The blood samples were centrifuged at 3,000 rpm for 15 minutes at room temperature to achieve serum specimens. The serum was separated to determine the amounts of neopterin using enzyme-linked immunosorbent assay (ELISA), Trp, and Kyn by HPLC.
Serum neopterin concentrations were determined using commercial ELISA kits (IBL) as directed by the manufacturer. The optical density was determined using a microplate reader at 450 nm (Spectra Max M2). The serum neopterin results were presented as nmol/L.
The HP Agilent 1100 HPLC system (Agilent) was used to detect serum Trp and Kyn levels using a reversed-phase C18. After protein precipitation with perchloric acid, Trp levels were evaluated using fluorescence detection at 285 nm excitation and 360 nm emission wavelengths. Kyn levels were assessed simultaneously using 360 nm UV absorption [14,15]. Serum Trp and serum Kyn levels were used to calculate IDO-1 enzyme activity and presented as µmol kynurenine to mmol Trp.
The standardized procedures employed by the central biochemistry laboratory of the hospital were used to determine blood concentrations of cardiac troponin, creatine kinase-myocardial band (CK-MB) natriuretic peptide (prohormone- B type natriuretic peptide, pro-BNP), CRP, lactate, LDL, urea, and creatinine.
Statistical Analysis
IBM SPSS ver. 23 (IBM Corp.) was used for the statistical analysis. Histogram visualizations and the Kolmogorov-Smirnov test assessed the variables' conformance to the normal distribution. When presenting descriptive analyses, mean, standard deviation, median, and minimum-maximum data were employed and compared with Pearson's chi-square and Fisher's exact tests. The t-test was utilized in independent groups, and the analysis of variance test was used when comparing more than two groups. The Kruskal-Wallis test assessed group differences, and the Mann-Whitney U-test assessed independent subgroups. Spearman's correlations were utilized to evaluate relationships between parameters and biochemical results. All tests were two-sided, and statistical significance was defined as a P-value less than 0.05.
RESULTS
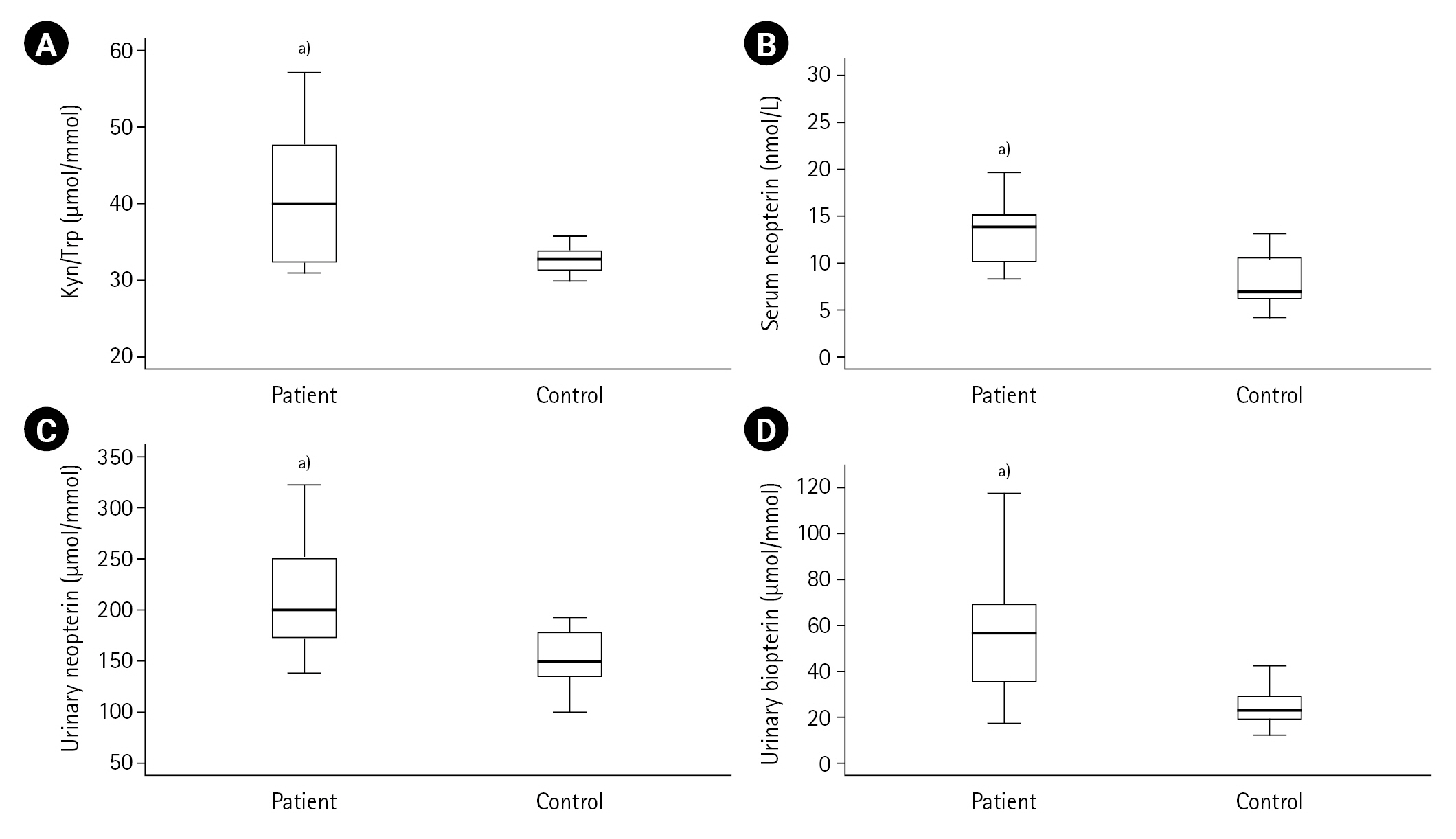
First, the measured values in the ACS patient (n=50) and control (n=20) groups were compared. Cardiac troponin, CK-MB, pro-BNP, lactate, LDL cholesterol, urea, creatinine, Kyn, Kyn/Trp, serum and urinary neopterin and urinary biopterin values were found to be higher in the ACS patient group compared to the control group (all P<0.001) as shown in Table 1, and Figures 2 and 3. The serum neopterin levels in the control groups were 7.7 ± 2.5 nmol/L and 13.8 ± 4.6 nmol/L in the patients' group. Moreover, Kyn/Trp ratio in the control and ACS patient groups was calculated as 30.8±5.1 and 45.5±12.2 μmol/mmol, respectively. The patient's urine neopterin levels were higher than controls, 220.1± 61.9 and 147.3±29.0 μmol/mol creatinine, respectively (P<0.001). Urinary biopterin levels were higher in ACS patients than controls, 245.9±75.0 and 137.0±37.4 μmol/mol creatinine, respectively (P<0.001).
The study patients were divided into two groups according to their ST elevations: STEMI (n=30) and NSTEMI (n=20). Between control and STEMI groups, significant differences were observed in CK-MB, lactate and kynurenine levels (all P<0.001). In contrast, these differences were not found between the control and NSTEMI groups (all P>0.001). There were significant differences in troponin, Kyn/Trp, serum neopterin, urinary neopterin and biopterin levels comparing control and STEMI or NSTEMI groups (all P<0.001). The serum neopterin levels in the STEMI group were 13.43±4.90 nmol/L; in the NSTEMI group, they were 14.37±4.24 nmol/L (P=0.320). Kyn/Trp in STEMI and NSTEMI groups were not significantly different and calculated as 44.58±10.43 and 46.89±14.94 μmol/mmol, respectively. NSTEMI and STEMI urine neopterin levels were 226.95±66.90 and 215.36±59.19 μmol/mol creatinine, respectively (P=0.654). Urinary biopterin levels in NSTEMI and STEMI were 225.06±54.24 and 259.84±84.27 μmol/mol creatinine, respectively (P=0.167). Table 2 shows no significant difference between STEMI and NSTEMI subgroups on serum pro-BNP, CRP, lactate, LDL, urea and creatinine, Trp and Kyn mean levels (all P>0.001). The other cardiac biomarkers, troponin and CK-MB expected to be higher in ACS patients (troponin 2.2±4.9 μg/L, CK-MB 72.8±108.1 UI/L) than controls (troponin 0.0±0.0 μg/L, CK-MB 29.3±19.8 UI/L) (both P<0.001). There was not any difference in comparisons of the measurements between ACS patients with STEMI and NSTEMI groups except creatine kinase. A significant difference only was observed in CK-MB levels (P=0.032), and the mean creatine kinase levels in the STEMI group (94.0±135.5 IU/L) were higher than in the NSTEMI group (40.9±17.3 IU/L).
The correlation of the measured values was investigated in the controls, and a positive correlation was found between Kyn/Trp and pro-BNP (r=0.456, P=0.05). There were positive correlations between serum Kyn-urea (r=0.357, P=0.019), serum Kyn-creatinine (r=0.381), Kyn/Trp-urea (r=0.358), serum neopterin-LDL cholesterol (r=0.318) and serum neopterin-urea (r=0.445) in patients with ACS. A negative correlation was observed between serum neopterin and lactate in patients with ACS (r=–0.366, P=0.022). In the STEMI group, there were positive correlations between LDL cholesterol and Kyn/Trp (r=0.459), serum Kyn-urea (r=0.410), serum Kyn-creatinine (r=0.421), and serum neopterin-creatinine (r=0.196) (all P<0.05). The correlation of the measured values in the NSTEMI group was also evaluated. A negative correlation between serum neopterin and lactate (r=–0.632, P=0.015) and a positive correlation between serum neopterin and urea (r=0.632, P=0.015) were observed.
DISCUSSION
Cardiovascular diseases are responsible for almost one-third of all fatalities worldwide, with ischemic heart disease accounting for 7.5 million deaths. ACS and sudden death account for the majority of ischemic heart disease-related deaths, with 1.8 million annually [16]. Stable biochemical markers are required to control the disease course and treatment efficacy. Troponin, CRP, pro-BNP, creatine kinase, LDL, serum urea, serum creatinine and lactate levels are routinely checked in emergency cardiac cases. In our study, the patients had high levels of the aforementioned parameters, except for CRP.
Neopterin is a valuable prognostic marker for risk classification in individuals with CAD. Susceptible atheromatous plaques and ACS have been shown to correlate with neopterin concentrations [17-19]. In acute myocardial infarction, as the fatal consequence of a plaque rupture, neopterin levels were significantly higher compared to patients with chronic CAD and control subjects. Gurumurthy et al. [20] concluded that neopterin levels in ACS patients were higher than in stable CAD patients, and serum neopterin levels were 11.5±3.2 nmol/L in STEMI and 9.8±2.9 nmol/L in NSTEMI patients. None of the measured biochemical parameters, including troponin and neopterin, can solely differentiate between STEMI and NSTEMI sub-groups. Zouridakis et al. [21] revealed that elevated neopterin concentrations are associated with faster progression of coronary atherosclerotic disease in patients with stable angina. Garcia-Moll et al. [22] found that individuals with unstable angina had greater detectable levels of neopterin than those with stable angina, suggesting that this molecule may have a role in plaque instability. According to this theory, the same researchers reported that neopterin levels were related to the number of complex lesions but not the degree and extent of coronary atherosclerosis in patients with unstable angina who underwent coronary angiography. These data indicate that neopterin may be a marker of "activity" rather than severity in coronary disease.
Neopterin strongly predicts unfavorable coronary events, particularly in patients with heart diseases [23,24]. However, the potential link between neopterin levels and the degree of coronary atherosclerosis is being debated due to conflicting research findings. For example, Tanaka et al. [25] found that higher neopterin concentrations were highly related to the severity of coronary atherosclerosis in people with stable angina pectoris. On the other hand, Schumacher et al. [26] found no significant difference in neopterin levels between individuals with mild and severe CAD. In another study, neopterin levels were found to be an independent biomarker of hospitalization for heart failure. To improve risk estimation, neopterin can be used with conventional biomarkers such as CRP and BNP [27]. In the present study, neopterin and pro-BNP values in ACS patients were significantly higher than in the control group. These results suggest that neopterin and pro-BNP may be used to predict heart failure hospitalization in cases with ACS.
In this study, the median values of serum neopterin concentrations were 13.7 nmol/L in the ACS patient group and 7.0 nmol/L in the control group (P<0.001). This statistically significant difference between the serum neopterin levels in the patient and control groups was also reflected in the neopterin excretion levels. As a result of this reflection, the mean urinary neopterin concentrations were found to be 220±62 μmol/mol creatinine in the patients with ACS and 147±29 μmol/mol creatinine in the control group (P<0.001). Both serum and urinary neopterin levels are higher in patients with ACS compared to the control group, and that it is positively correlated with CRP values indicates a T cell-mediated immune response.
The findings of this study indicated that the mean urinary biopterin concentrations were 246±75 μmol/mol creatinine in the ACS patient group and 137±37 μmol/mol creatinine in the control group (P<0.001). The statistically significant differences in neopterin levels in the study groups accompanied biopterin excretion. BH4 is a cofactor of the enzymatic hydroxylation of phenylalanine, tyrosine and Trp and is also known as a co-stimulator of lymphocyte activation. In a study performed, patients who underwent angiography were evaluated, and serum Trp, Kyn and neopterin concentrations were measured. As a result, the Kyn/Trp was correlated with CRP and neopterin levels [10]. The present study found a positive correlation between Kyn/Trp and serum urea. The results show that the IDO value may increase over time in correlation with serum urea, similar to the data previously reported by Murr et al. [28]. Serum neopterin concentration positively correlates with LDL cholesterol and serum urea and negatively correlates with lactate, suggesting a possible link between neopterin levels and atherosclerosis. These findings may also indicate a possible relationship between IDO activity, inflammation, and immunological activation.
Furthermore, the kynurenine pathway, inflammation, and immune activation may promote the effect of the inflammatory process in CAD [29]. In a study with a large number of elective coronary angiography patients, the Kyn/Trp was found to be a strong predictor of acute myocardial infarction, major cardiac event, and mortality with a small incremental prognostic value above that achieved by classical risk factors in patients referred for coronary angiography for suspected stable CAD [30]. All patients were followed for 6 months, and 3 were deceased in this period. The measured parameters in the samples collected at the beginning of the study were evaluated, and it was observed that the values of the dead patients (1/3 from STEMI, 2/3 from NSTEMI) were not within the extreme values. It means that potential changes in measured parameters were not useful in determining life expectancy in ACS caused by angiography atherosclerosis.
The limitation of the present study is the lack of monitoring biomarker levels during hospitalisation. Further, larger patient-based studies, including unstable angina patients, are needed to support these results. Increased levels of neopterin and dysregulated IDO activity in ACS patients can be considered a prognostic tool in evaluating the severity of atherosclerotic rupture. Along with classical biomarkers in the diagnosis and follow-up of cardiovascular diseases, emerging parameters like neopterin and Kyn/Trp will be helpful in acute and critical practice.
In conclusion, the elevations of cardiac biomarkers indicate myocardial damage, but do not highlight an infarction in the coronary artery. In case of changes in cardiac biomarkers, only ACS should not be considered, further differential diagnoses should be kept in mind. These findings are in line with the role of inflammation in CAD and the present study provides evidence that both neopterin and IDO activity are in accordance with immune and inflammatory states in ACS.
KEY MESSAGES
▪ The measured levels of neopterin, biopterin and the kynurenine to tryptophan ratio reflecting indoleamine 2,3-dioxygenase (IDO) activity, and the specifically known biomarkers such as cardiac troponin, creatine kinase, myoglobin, and natriuretic peptides are significantly higher in acute coronary syndrome (ACS) patients compared to controls.
▪ These measured parameters are not distinctive in the patients with ACS sub-grouped based on their electrocardiograms, such as ST-elevation and non-ST-elevation myocardial infarction cases.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This study was partially, TDK -2019-17670, supported by Hacettepe University.
-
AUTHOR CONTRIBUTIONS
Conceptualization: IK, TB. Methodology: GG, TB. Formal analysis: BK, GG. Funding acquisition: TB. Data curation: IK, SS. Project administration: TB. Writing–original draft: IK, SS. Writing–review & editing: GG, TB.
Acknowledgments
None.
Figure 1.The flowchart of the study. ACS: acute coronary syndrome; STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction.
Figure 2.Acute coronary syndrome parameters in patients and controls: (A) troponin-A, (B) creatine kinase-myocardial band (CK-MB), (C) prohormone-B type natriuretic peptide (pro-BNP), (D) C-reactive protein (CRP). a)P<0.001, vs. control group.
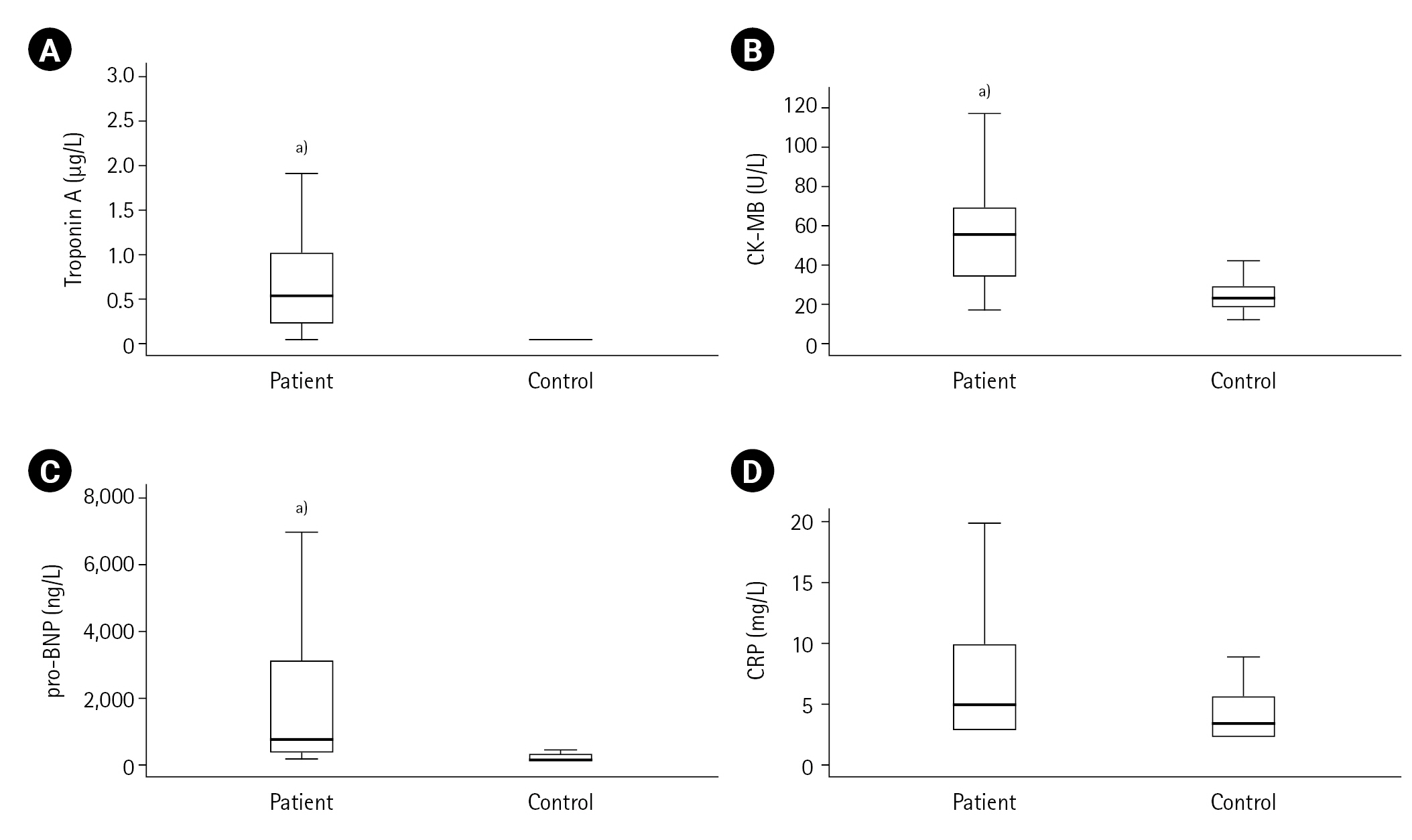
Figure 3.Kynurenine (Kyn)/tryptophan (Trp), neopterin and biopterin levels in acute coronary syndrome patients and controls. (A) Kyn/Trp, (B) Serum neopterin, (C) Urinary neopterin, (D) Urinary biopterin. a)P<0.001, vs. control group.
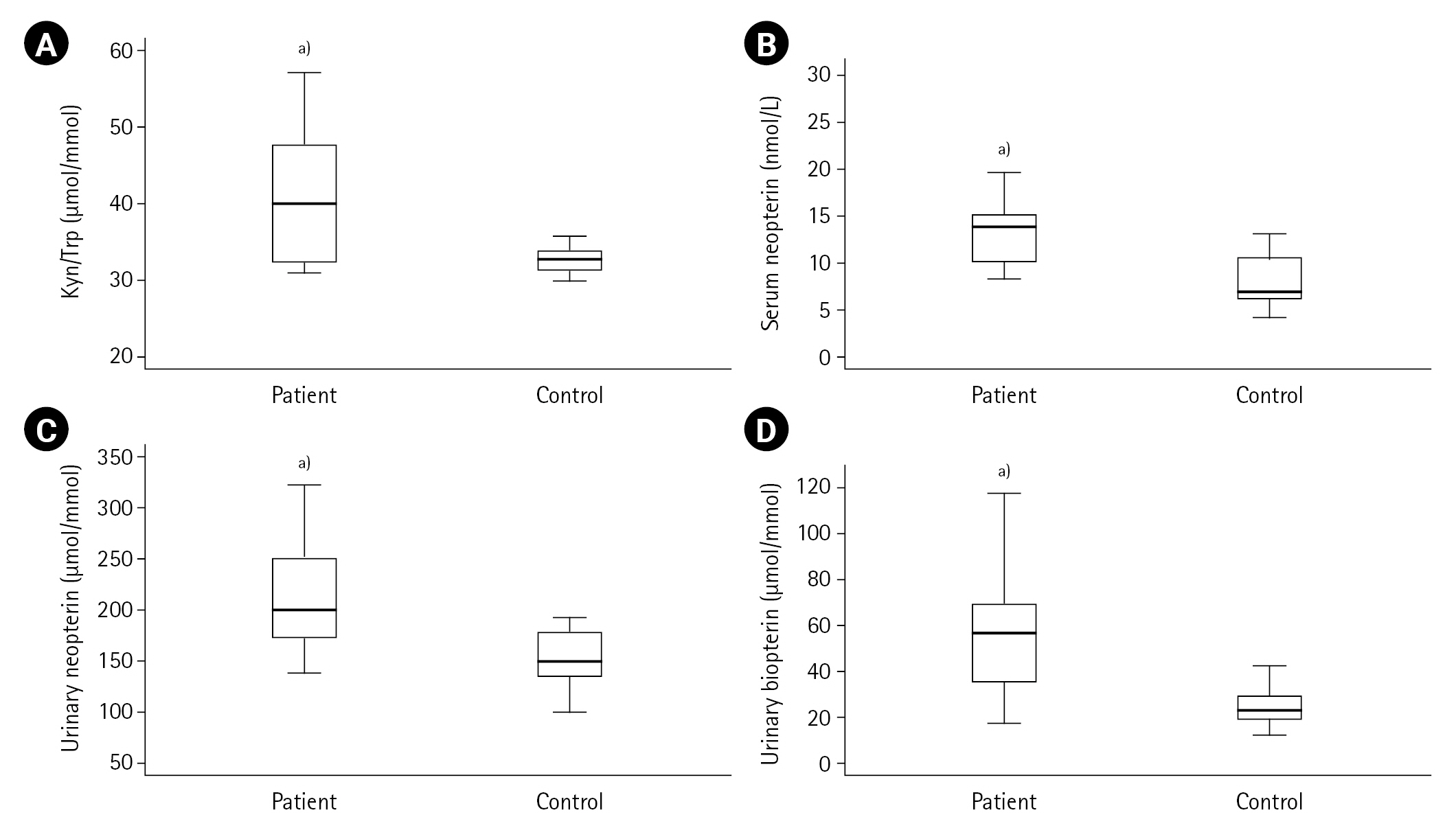
Table 1.Comparison of measured values between ACS patients and control groups (n=70)
|
Variable |
Control group (n=20) |
Patient group (n=50) |
P-value |
|
Age (yr) |
54 |
63 |
0.003a)
|
|
BMI (kg/m2) |
28.7±5.2 |
27.9±3.4 |
0.943b)
|
|
Ejection fraction (%) |
63.5±2.4 |
47.4±10.6 |
<0.001b)
|
|
pro-BNP (ng/L) |
126±65 |
3,083±6,365 |
<0.001b)
|
|
CRP (mg/L) |
9.1±12.8 |
14.3±28.6 |
1.000b)
|
|
Lactate (mmol) |
1.5±0.4 |
2.1±1.1 |
0.020b)
|
|
LDL (mg/dl) |
102.6±32.2 |
124.2±42.5 |
0.038b)
|
|
Urea (ng/dl) |
28.0±8.8 |
40.0±22.5 |
0.001b)
|
|
Creatinine, serum (ng/dl) |
0.7±0.2 |
1.2±1.3 |
<0.001b)
|
|
Tryptophan (μmol/L) |
56.5±10.4 |
52.3±11.0 |
0.160a)
|
|
Kynurenine (μmol/L) |
1.8±0.3 |
2.4±0. 6 |
<0.001b)
|
Table 2.Comparison of measured values between STEMI and NSTEMI subgroups
|
Variable |
ACS patient (n=50)
|
P-value |
|
STEMI (n=30) |
NSTEMI (n=20) |
|
Age (yr) |
62 |
65 |
0.276a)
|
|
BMI (kg/m2) |
28.5±3.9 |
27.1±2.4 |
0.217b)
|
|
Ejection fraction (%) |
47.2±10.1 |
47.7±11.6 |
0.740b)
|
|
pro-BNP (ng/L) |
2,351±4,081 |
4,181±8,773 |
0.882b)
|
|
CRP (mg/L) |
10.1±17.9 |
20.8±39.2 |
0.950b)
|
|
Lactate (mmol) |
2.2±1.2 |
1.8±0.7 |
0.433b)
|
|
LDL (mg/dl) |
125.3±40.3 |
122.5±46.6 |
0.921b)
|
|
Urea (ng/dl) |
34.9±9.9 |
47.6±32.5 |
0.120b)
|
|
Creatinine, serum (ng/dl) |
0.9±0.2 |
1.5±2.1 |
0.645b)
|
|
Tryptophan (μmol/L) |
49.8±10.4 |
56.3±11.1 |
0.054a)
|
|
Kynurenine (μmol/L) |
2.3±0.5 |
2.5±0.6 |
0.157b)
|
References
- 1. Brown RM. Acute coronary syndrome in women. Emerg Med Clin North Am 2022;40:629-36.ArticlePubMed
- 2. Atwood J. Management of acute coronary syndrome. Emerg Med Clin North Am 2022;40:693-706.ArticlePubMed
- 3. Simons M, Alpert JS. Acute coronary syndrome: terminology and classification [Internet]. UpToDate. 2022;[cited 2023 Jul 20]. Available from: https://www.uptodate.com/contents/acute-coronary-syndrome-terminology-and-classification.
- 4. Pacileo M, Cirillo P, De Rosa S, Ucci G, Petrillo G, Musto D’Amore S, et al. The role of neopterin in cardiovascular disease. Monaldi Arch Chest Dis 2007;68:68-73.ArticlePubMedPDF
- 5. Ünüvar S, Erge D, Kılıçarslan B, Gözükara Bağ HG, Çatal F, Girgin G, et al. Neopterin levels and indoleamine 2,3-dioxygenase activity as biomarkers of immune system activation and childhood allergic diseases. Ann Lab Med 2019;39:284-90.ArticlePubMedPMCPDF
- 6. Cavaleri D, Bartoli F, Capogrosso CA, Guzzi P, Moretti F, Riboldi I, et al. Blood concentrations of neopterin and biopterin in subjects with depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2023;120:110633. ArticlePubMed
- 7. Hoekstra R, van den Broek WW, Fekkes D, Bruijn JA, Mulder PG, Pepplinkhuizen L. Effect of electroconvulsive therapy on biopterin and large neutral amino acids in severe, medication-resistant depression. Psychiatry Res 2001;103:115-23.ArticlePubMed
- 8. Sabuncuoğlu S, Öztaş Y, Yalcinkaya A, Ünal S, Baydar T, Girgin G. The increased neopterin content in Turkish pediatric patients with sickle cell anemia. Ann Hematol 2020;99:41-7.ArticlePubMedPDF
- 9. Gürcü S, Girgin G, Yorulmaz G, Kılıçarslan B, Efe B, Baydar T. Neopterin and biopterin levels and tryptophan degradation in patients with diabetes. Sci Rep 2020;10:17025. ArticlePubMedPMCPDF
- 10. Ozkan Y, Sukuroglu MK, Tulmac M, Kisa U, Simsek B. Relation of kynurenine/tryptophan with immune and inflammatory markers in coronary artery disease. Clin Lab 2014;60:391-6.ArticlePubMed
- 11. Swardfager W, Herrmann N, Dowlati Y, Oh PI, Kiss A, Walker SE, et al. Indoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery disease. Psychoneuroendocrinology 2009;34:1560-6.ArticlePubMed
- 12. Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, et al. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest 2003;33:550-4.ArticlePubMedPDF
- 13. Moe KT, Wong P. Current trends in diagnostic biomarkers of acute coronary syndrome. Ann Acad Med Singap 2010;39:210-5.ArticlePubMed
- 14. Girgin G, Sahin TT, Fuchs D, Yuksel O, Kurukahvecioglu O, Sare M, et al. Tryptophan degradation and serum neopterin concentrations in intensive care unit patients. Toxicol Mech Methods 2011;21:231-5.ArticlePubMed
- 15. Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem 1997;43:2424-6.ArticlePubMedPDF
- 16. Matsushita K, Yatsuya H, Tamakoshi K. Epidemiology of acute coronary syndrome. Nihon Rinsho 2006;64:625-32.
- 17. van Haelst PL, Liem A, van Boven AJ, Veeger NJ, van Veldhuisen DJ, Tervaert JW, et al. Usefulness of elevated neopterin and C-reactive protein levels in predicting cardiovascular events in patients with non-Q-wave myocardial infarction. Am J Cardiol 2003;92:1201-3.ArticlePubMed
- 18. Kaski JC, Consuegra-Sanchez L, Fernandez-Berges DJ, Cruz-Fernandez JM, Garcia-Moll X, Marrugat J, et al. Elevated serum neopterin levels and adverse cardiac events at 6 months follow-up in Mediterranean patients with non-ST-segment elevation acute coronary syndrome. Atherosclerosis 2008;201:176-83.ArticlePubMed
- 19. Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez M. Usefulness of neopterin levels and left ventricular function for risk assessment in survivors of acute myocardial infarction. Int J Cardiol 2006;111:318-20.ArticlePubMed
- 20. Gurumurthy P, Borra SK, Yeruva RK, Babu S, Thomas J, Cherian KM. Estimation of serum neopterin in patients with acute coronary syndrome. Asian Cardiovasc Thorac Ann 2013;21:426-31.ArticlePubMedPDF
- 21. Zouridakis E, Avanzas P, Arroyo-Espliguero R, Fredericks S, Kaski JC. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation 2004;110:1747-53.ArticlePubMed
- 22. Garcia-Moll X, Coccolo F, Cole D, Kaski JC. Serum neopterin and complex stenosis morphology in patients with unstable angina. J Am Coll Cardiol 2000;35:956-62.ArticlePubMed
- 23. Avanzas P, Arroyo-Espliguero R, Quiles J, Roy D, Kaski JC. Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur Heart J 2005;26:457-63.ArticlePubMed
- 24. Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, Quiles J, Zouridakis E, Kaski JC. Prognostic value of neopterin levels in treated patients with hypertension and chest pain but without obstructive coronary artery disease. Am J Cardiol 2004;93:627-9.ArticlePubMed
- 25. Tanaka T, Nakamura Y, Nasuno A, Mezaki T, Higuchi K, Fukunaga H, et al. Plasma concentrations of monocyte chemoattractant protein 1 (MCP-1) and neopterin in the coronary circulation of patients with coronary artery disease. Circ J 2004;68:114-20.ArticlePubMed
- 26. Schumacher M, Halwachs G, Tatzber F, Fruhwald FM, Zweiker R, Watzinger N, et al. Increased neopterin in patients with chronic and acute coronary syndromes. J Am Coll Cardiol 1997;30:703-7.ArticlePubMed
- 27. Nazer B, Ray KK, Sloan S, Scirica B, Morrow DA, Cannon CP, et al. Prognostic utility of neopterin and risk of heart failure hospitalization after an acute coronary syndrome. Eur Heart J 2011;32:1390-7.ArticlePubMed
- 28. Murr C, Grammer TB, Kleber ME, Meinitzer A, März W, Fuchs D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur J Clin Invest 2015;45:247-54.ArticlePubMed
- 29. Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, et al. Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 2007;115:3071-8.ArticlePubMed
- 30. Pedersen ER, Svingen GF, Schartum-Hansen H, Ueland PM, Ebbing M, Nordrehaug JE, et al. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J 2013;34:2689-96.ArticlePubMed
Citations
Citations to this article as recorded by

- Biomarkers to monitor the prognosis, disease severity, and treatment efficacy in coronary artery disease
Armand N. Yazdani, Michaela Pletsch, Abraham Chorbajian, David Zitser, Vikrant Rai, Devendra K. Agrawal
Expert Review of Cardiovascular Therapy.2023; 21(10): 675. CrossRef - Evaluation of Neopterin as a Neuroinflammatory Marker for Peripheral Neuropathy in Type 2 Diabetic Patients
Israa Abdelmalik Salem, Sura Ahmed Abdulsattar, Haider Fadhil Alrubaye
Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 ).2023; 5(1S): S183. CrossRef
 , Sonia Sanajou1
, Sonia Sanajou1 , Bilge Kilicarslan1
, Bilge Kilicarslan1 , Gözde Girgin1
, Gözde Girgin1 , Terken Baydar1
, Terken Baydar1







 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite