Abstract
-
Background
- Delirium in critically ill children can result in long-term morbidity. Our main objectives were to evaluate the effectiveness of a new protocol on the reduction, prevalence, and duration of delirium and to identify associated risk factors.
-
Methods
- The effectiveness of the protocol was evaluated by a chart review in all critically ill children aged 1 month to 15 years during the study period. A Cornell Assessment of Pediatric Delirium score ≥9 was considered positive for delirium. Data on delirium prevalence and duration from the pre-implementation and post-implementation phases were compared. Univariate and multivariate analyses were used to identify the risk factors of delirium.
-
Results
- A total of 120 children was analyzed (58 children in the pre-implementation group and 62 children in the post-implementation group). Fifty children (41.7%) screened positive for delirium. Age less than 2 years, delayed development, use of mechanical ventilation, and pediatric intensive care unit (PICU) stay >7 days were significantly associated with delirium. The proportion of children screened positive was not significantly different after the implementation (before, 39.7% vs. after, 43.5%; P=0.713). Subgroup analyses revealed a significant reduction in the duration of delirium in children with admission diagnosis of cardiovascular problems and after cardiothoracic surgery.
-
Conclusions
- The newly implemented protocol was able to reduce the duration of delirium in children with admission diagnosis of cardiovascular problems and after cardiothoracic surgery. More studies should be conducted to reduce delirium to prevent long-term morbidity after PICU discharge.
-
Keywords: children; delirium; intensive care; morbidity
INTRODUCTION
Delirium is an acute cerebral dysfunction that can be caused by systemic illness or a medical treatment, especially in a critical care setting. It is sometimes referred to as intensive care unit psychosis or toxic psychosis [1,2]. According to the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) in 2015, delirium is also defined as disturbances in consciousness, attention, perception, thinking, memory, emotion, and sleep-wake schedule [3]. The prevalence rate of delirium in critically ill children ranges from 10% to 56%, with higher rates among children younger than 2 years [1-2,4-10].
Delirium can be classified into three subtypes: hyperactive, hypoactive, or mixed [1-2,11]. Children experiencing delirium have more frequent severe perceptual disturbances, mood lability, agitation, and visual hallucinations [11]. Critically ill children with delirium are associated with longer pediatric intensive care unit (PICU) and hospital stays, neurocognitive dysfunction, post-traumatic stress disorder, and higher rates of mortality [1,10]. Moreover, with a longer duration of delirium, more frequent severe cognitive and memory problems develop among children surviving critical illness [12,13]. Thus, delirium in critically ill children is a major health issue that can result in long-term morbidity.
Studies by Patel et al. in 2017 [14] and Jesus et al. [15] in 2020 suggested systematic approaches for the management of delirium in children who are critically ill. The first step of these approaches is to recognize and manage the underlying conditions and iatrogenic factors leading to delirium. Then, perform a stepwise approach using environmental modification, nonpharmacologic intervention, and then pharmacologic intervention [14,15]. A mnemonic known as “BRAIN MAPS” is used as a part of the assessment, evaluation, and treatment in the delirium clinical pathway and is recommended by the Society of Critical Care Medicine. BRAIN MAPS involves bringing oxygen (B), reducing or removing deliriogenic drugs such as benzodiazepines or anticholinergics (R), atmosphere modification (A), infection/inflammation control and reducing immobilization (I), treatment of new organ dysfunction and metabolic disturbances (NM), awakening schedule (A), pain (P), and sedation control (S) [16-18]. There is limited evidence of the effectiveness of such approaches in reducing delirium in critically ill children. Furthermore, there is no local guideline in Thailand on the management of delirium in critically ill children. Thus, the main objective of this study was to evaluate the effectiveness of the newly devised delirium care map protocol in the reduction of delirium in critically ill children in terms of both prevalence and duration, PICU stay, and ventilator days. The secondary objective was to identify the risk factors associated with delirium in critically ill children.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of Thammasat University Hospital, Thammasat University, Thailand (No. MTU-EC-PE-0-246/65). Informed consent was waived due to the retrospective review of the implemented protocol.
Study Design and Participants
A chart review of all children aged 1 month to 15 years who were admitted to the PICU from March to October 2022 was conducted. Our PICU is a mixed medical-surgical tertiary, single-patient room intensive care unit that receives approximately 25–30 patients per month. The nurse-to-patient ratio is 1:1 or 1:2 based on the severity of the patient’s condition.
Intervention
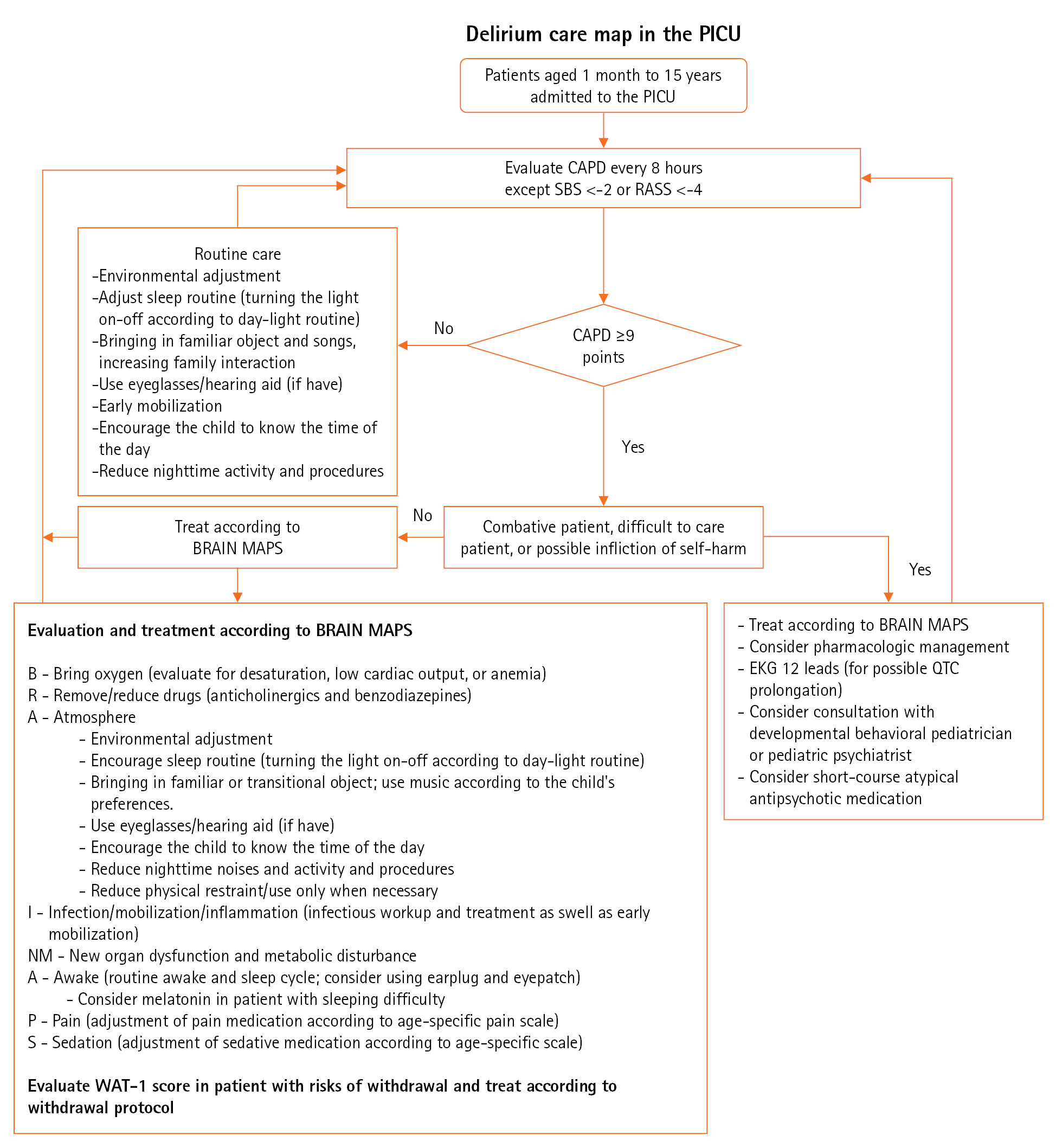
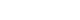
From March 2022, the Cornell Assessment of Pediatric Delirium (CAPD) has been used as a screening tool for delirium in our critical care unit. The CAPD was conducted in all patients admitted to the PICU every 8 hours at the start of the nursing shift. Before this, no screening tool for delirium was used in the unit. All pediatric critical care nurses were trained by attending pediatric intensivists and senior critical care nurses who were familiar with the screening tool. Good interrater reliability of 0.85 was obtained before initiation of the routine assessment. The CAPD is a valid screening tool with excellent sensitivity and specificity for critically ill children of all ages and can be conducted in patients with developmental delays. CAPD consists of eight questions aiming to assess consciousness, cognition, orientation, psychomotor activity, and affect/distress. The score was given on a Likert scale of 0–4 (0=always, 1=often, 2=sometimes, 3=rarely, and 4=never) based on the assessment. The CAPD is faster, needing less than 2 minutes to complete [1]. A CAPD score ≥9 is considered positive for delirium. Deeply sedated patients (Richmond Agitation-Sedation scale [RASS] <–4 or State Behavioral Scale [SBS] <–2) were not assessed until awake. RASS is a 10-point scale for the evaluation of agitation and sedation ranging from +4 (combative) to –5 (unarousable) [19]. The SBS is also used for evaluation of sedation and agitation in mechanically ventilated children in our unit. This is a 6-point scale ranging from +2 (agitated) to –3 (unresponsive) [20]. These scales are currently used as a standard practice for evaluation of sedation and agitation in our PICU (RASS in non-mechanically ventilated children, SBS in mechanically ventilated children).
The delirium care map protocol based on the mnemonic “BRAIN MAPS” was outlined as a quality improvement project of the hospital by pediatric intensivists and pediatric critical care nurses in June 2022 to decrease the proportion and duration of patients with delirium (Figure 1). The components of the protocol mostly focus on the non-pharmacologic management of delirium, adjustment of the PICU environment, and treatment of the underlying conditions. Pharmacologic treatment of delirium was reserved only for patients who were combative or at risk of self-harm. A 1-month training period was required before implementation to ensure consistency and compliance with the protocol. Thus, the patients who were admitted from March 1, 2022, to June 30, 2022, were considered as a pre-implementation group, and the patients admitted from July 1, 2022, to October 31, 2022, served as the post-implementation group. To ensure the effectiveness of the protocol, screening and protocol compliance were randomly assessed. All demographic data, as well as admission diagnosis, underlying comorbidities, delirium duration, PICU duration, sedation, the neuromuscular blocking agent used, and ventilator days, were recorded.
Statistical Analyses
Delirium duration was calculated as the number of shifts with CAPD ≥9 points multiplied by 8 hours (shift duration). For instance, if a child screened positive for delirium 10 times during the PICU stay, the duration was recorded as 80 hours. Delayed development was defined using the red flags described by the American Academy of Pediatrics [21]. All demographic data, PICU length of stay, and ventilator days were analyzed using descriptive statistics. The prevalence of delirium was reported as a percentage. Data on delirium from the pre-implementation and post-implementation phases were compared with the expectation of reductions in delirium rates and duration. The Mann-Whitney U-test was used to compare quantitative data among intervention groups. Univariate analyses were used to identify the risk factors of delirium. Statistically significant factors from the univariate analyses were used for the adjustment during multivariate analyses. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS ver. 24 (IBM Corp.).
RESULTS
Demographic Data
The demographic data are presented in Table 1. A total of 120 children was admitted to the PICU during the study period. All children were included in the analysis. Fifty-eight children were allocated to the pre-implementation group and the remaining 62 children were allocated to the post-implementation group (Figure 2). The median age for the overall cohort was 3.1 years (interquartile range [IQR], 0.9–6.8), and 43 children (35.8%) were younger than 2 years. Approximately 56.7% of the participants were male.
Cardiovascular comorbidity served as the main underlying condition in the cohort (41.7%), followed by neurologic comorbidity (7.5%) and gastrointestinal comorbidity (5.8%). A total of 37 children (30.8%) had developmental delays and 56 children (46.7%) were admitted to the PICU for postoperative care. Among the children admitted for postoperative care, the major type of surgery was cardiothoracic surgery (32/56, 57%). The most common admission diagnoses were cardiovascular (38.2%) and gastrointestinal (13.3%) problems. The median Pediatric Risk for Mortality (PRISMI) III score [22] was 2.0 (IQR, 0.0–5.0). Inotropic medications were used in 47 children (39.2%) with a median vasoactive inotropic score (VIS) [23] of 14.0 (IQR, 7.0–24.0). Extracorporeal membrane oxygenator support (ECMO) was compulsory in four children (3.3%), one (1.7%) during the pre-implementation phase, and three (4.8%) in the post-implementation phase. A total of 48 children (40.0%) was mechanically ventilated with a median duration of 2.5 days (IQR, 1.0–8.8 days). Continuous infusion of sedatives and neuromuscular blocker (NMB) were used in 42 (35.0%) and nine (7.5%) children, respectively. Five children (4.2%) exhibited signs of withdrawal. The median PICU stay was 3.0 days (IQR, 1.0–8.0 days), and 35 children (29.2%) required PICU admission for greater than 7 days. Three children (2.5%) died during the study period. The overall demographic data including age, gender, underlying condition, admission diagnoses, developmental status, PRISM III score, VIS, inotropic support, ECMO support, respiratory support, sedation use, NMB use, and withdrawal were not significantly different between intervention groups.
Outcomes of Delirium Care Protocol
The compliance rate of the screening was 95%. The bundle compliance was randomly examined and found to be 70%. The most difficult components with which to comply were a sleeping routine and reduction of night-time noises. Parental visits were limited to 30 minutes each day to comply with the hospital policy. A total of 50 children (41.7%) had positive delirium screening. The median CAPD score for the overall cohort was 7.0 (3.0–12.0). The proportion of children with positive CAPD screening was not significantly different among groups (39.7% during the pre-intervention phase vs. 43.5% during the post-intervention phase; P=0.713). The median delirium duration during the post-intervention phase seemed to be shorter than the pre-intervention phase, but the difference was not significant (32.0 hours [IQR, 18.0–8.0] vs. 56.0 hours [IQR, 32.0–72.0], P=0.274). No children received atypical antipsychotics during the study period. The median CAPD score, mechanical ventilator support days, PICU stays, and mortality rates were not statistically different between the groups (Table 1).
In a subgroup analysis of children with the admission diagnosis of cardiovascular problems, there was a significant reduction in the duration of delirium after implementation of the protocol, from 64.0 hours (IQR, 54–80 hours) to 32.0 hours (IQR, 22–68 hours) (P=0.001). A similar result was found in children after cardiothoracic surgery (60.0 hours [IQR, 50–70) vs. 28.0 hours [IQR, 24–58), P=0.02).
Factors Associated with Positive Delirium Screening
Table 2 outlines the factors associated with delirium among participants. The proportion of children younger than 2 years was significantly higher in the positive delirium screening group (48.0% vs. 27.1%, P=0.022). There was also a significantly larger proportion of children with delayed development in the delirium group (52.0% vs. 15.7%, P<0.001). Children with positive screening for delirium had significantly higher median PRISM III scores, median CAPD scores, longer mechanical ventilation support days, and longer PICU stays. All children who required ECMO and NMB had positive delirium screening. Withdrawal was only found in children with delirium. The proportions of children with admission diagnosis of cardiovascular problems, who required mechanical ventilation, continuous infusion of sedation, PICU stay >7 days, and mechanical ventilation >7 days were significantly larger in the delirium group. Median age, sex, underlying comorbidity, postoperative status, and mortality were not significantly different among groups.
The univariate and multivariate analyses of associating factors are presented in Tables 3 and 4. Age younger than 2 years, delayed development, admission diagnosis of cardiovascular problem, inotropic support, mechanical ventilator, mechanical ventilation >7 days, use of sedation and NMB, and PICU stay >7 days were associated with positive delirium screening (P<0.05) according to univariate analyses. After adjustment for significant factors, age younger than 2 years, delayed development, and PICU stay >7 days were associated with positive delirium screening with adjusted odds ratio (aOR) of 3.41 (95% confidence interval [CI], 1.19–9.73), 5.03 (95% CI, 1.73–14.59), and 11.58 (95% CI, 2.90–46.23), respectively.
DISCUSSION
The prevalence rate of delirium in this cohort was substantially high at 41.7%. This was comparable to previous studies, with the prevalence of delirium ranging from 10% to 57% [1,2,4-10]. We also demonstrated that age less than 2 years, delayed development, use of mechanical ventilation, and a PICU stay >7 days were significantly associated with delirium in critically ill children. These findings were consistent with the previously reported studies by Silver et al. [5] and Traube et al. [1].
A large study in critically ill adults revealed that the multimodal ABCDEF bundle was associated with a lower likelihood of delirium with an aOR of 0.60 (95% CI, 0.49–0.72) [24]. There were limited data on the effectiveness of the multimodal protocol for the management of delirium in critically ill children [25-27]. The implementation of our new delirium protocol was unable to significantly reduce the prevalence and duration of delirium in the overall study cohort. Nevertheless, in the subgroup analysis of children with the admission diagnosis of cardiovascular problems, there was a significant decline in the duration of delirium from 64.0 hours (IQR, 54–80 hours) to 32 hours (IQR, 22–68 hours) (P=0.001). The same was found in children after cardiothoracic surgery (60.0 hours [IQR, 50–70] vs. 28.0 hours [IQR, 24–58], P=0.02). Several studies have demonstrated that young children are among the most vulnerable groups to develop delirium during critical illness, especially after cardiothoracic surgery [28,29]. Similar findings were demonstrated in a recent study by Michel et al. [25], outlining a non-significant reduction of delirium prevalence after implementation of the delirium bundles but a significant reduction among children after cardiothoracic surgery. Unlike in our study, Michel et al. [25] showed a significant reduction of delirium in subgroups of children younger than 5 years. We were only able to illustrate a reduction in the duration of delirium in children with an admission diagnosis of cardiovascular problems and those after cardiothoracic surgery. This might be due to the complex environment in the PICU setting and the multifactorial etiologies of delirium. Nevertheless, a reduction in the duration of delirium could alleviate the possible cognitive and memory problems after PICU discharge [12,13].
A prolonged stay in the PICU with the use of sedation and mechanical ventilation was associated with the development of delirium [1,5]. Approximately 29.2% of children in our cohort required a PICU stay >7 days, 40% required mechanical ventilation, and 35% required continuous infusion of sedation. Furthermore, children with positive screening for delirium had significantly higher median PRISM III scores, signifying the higher severity and complexity of the conditions. These factors might lead to continuous exposure of factors leading to delirium. Another factor limiting the effectiveness of the delirium protocol might be the pre-existing early mobilization protocol within the unit. A study by Simone et al. [27] showed that sequential implementation of delirium, sedation, and early mobility protocol can reduce the prevalence of delirium. An early mobilization protocol was implemented at our center in June 2020, and might have obscured the magnitude of the overall protocol effectiveness.
This study was one of the first aiming to reduce the prevalence of delirium in critically ill children by implementing the delirium care map protocol. A major strength of this study was that the compliance rate of the screening was as high as 95%. Furthermore, the compliance rate of the patients randomly examined every month was also high at approximately 70%. This compliance rate was comparable with the study by Michel et al. [25] (compliance screening rate of 95% and bundle adherence rate of 72%) and significantly higher than the study by Franken et al. [26] (compliance screening rate of 9%; no report on the compliance rate of the protocol). With the high compliance rate in both screening and protocol adherence, the true effectiveness of the protocol could be evaluated. Nonetheless, the most difficult protocol components to comply with were the reduction of night-time noises and encouragement of a sleep routine. Due to the nature of the university hospital, night-time admission and procedures as well as noises were difficult to control, regardless of the single-room patient status, which might compromise the effectiveness of the protocol. Furthermore, the limited time for a parental visit might also play a role in a non-significant change in the prevalence of delirium.
The other limitations of this study should also be outlined. This was a single-center study in a tertiary care university hospital. Thus, it might not be generalized to all hospital settings. A limited sample size within this cohort made it difficult to perform a subgroup analysis in different groups of children with different conditions. Nevertheless, this sample size was adequately powered for a reduction in the prevalence of delirium by 20% with β error of 0.2 and alpha error of 0.05. Regardless of these limitations, this is a pilot study for larger multicenter studies. More studies, especially multicenter studies, are also warranted to evaluate long-term cognitive decline and memory problems in children with delirium, as well as the validation and long-term effectiveness of the protocol.
Delirium is substantially prevalent among critically ill children in our cohort. The newly implemented protocol was able to reduce the duration of delirium in children with an admission diagnosis of cardiovascular problems and children after cardiothoracic surgery. More studies should be conducted to reduce delirium and prevent long-term morbidity in critically ill children after PICU discharge.
KEY MESSAGES
▪ A total of 50 children (41.7%) screened positive for delirium.
▪ Age less than 2 years, delayed development, use of mechanical ventilation, and pediatric intensive care unit stay >7 days were significantly associated with delirium.
▪ The proportion of children with positive screening was not significantly different after the implementation (before, 39.7% vs. after, 43.5%; P=0.713).
▪ Subgroup analyses revealed a significant reduction in the duration of delirium in children with an admission diagnosis of cardiovascular problems and after cardiothoracic surgery.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
This study was supported by the Research Group in Pediatric Care of the Faculty of Medicine, Thammasat University.
-
AUTHOR CONTRIBUTIONS
Conceptualization: CC. Methodology: CC. Formal analysis: CC. Data curation: all authors. Visualization: CC. Project administration: all authors. Funding acquisition: CC. Writing–original draft: all authors. Writing–review & editing: all authors.
Acknowledgments
The authors would like to show appreciation to all the residents, faculty, and nursing staff of our hospital as well as the Research Group for Pediatric Care of the Faculty of Medicine, Thammasat University for their strong support and extensive cooperation in making this project successful.
Figure 1.Protocol for delirium care of critically ill children. PICU: pediatric intensive care unit; SBS: State Behavioral Scale; RASS: Richmond Agitation-Sedation Scale; CAPD: Cornell Assessment of Pediatric Delirium; WAT-1: withdrawal assessment tool; EKG: electrocardiogram; QTC: corrected QT interval.
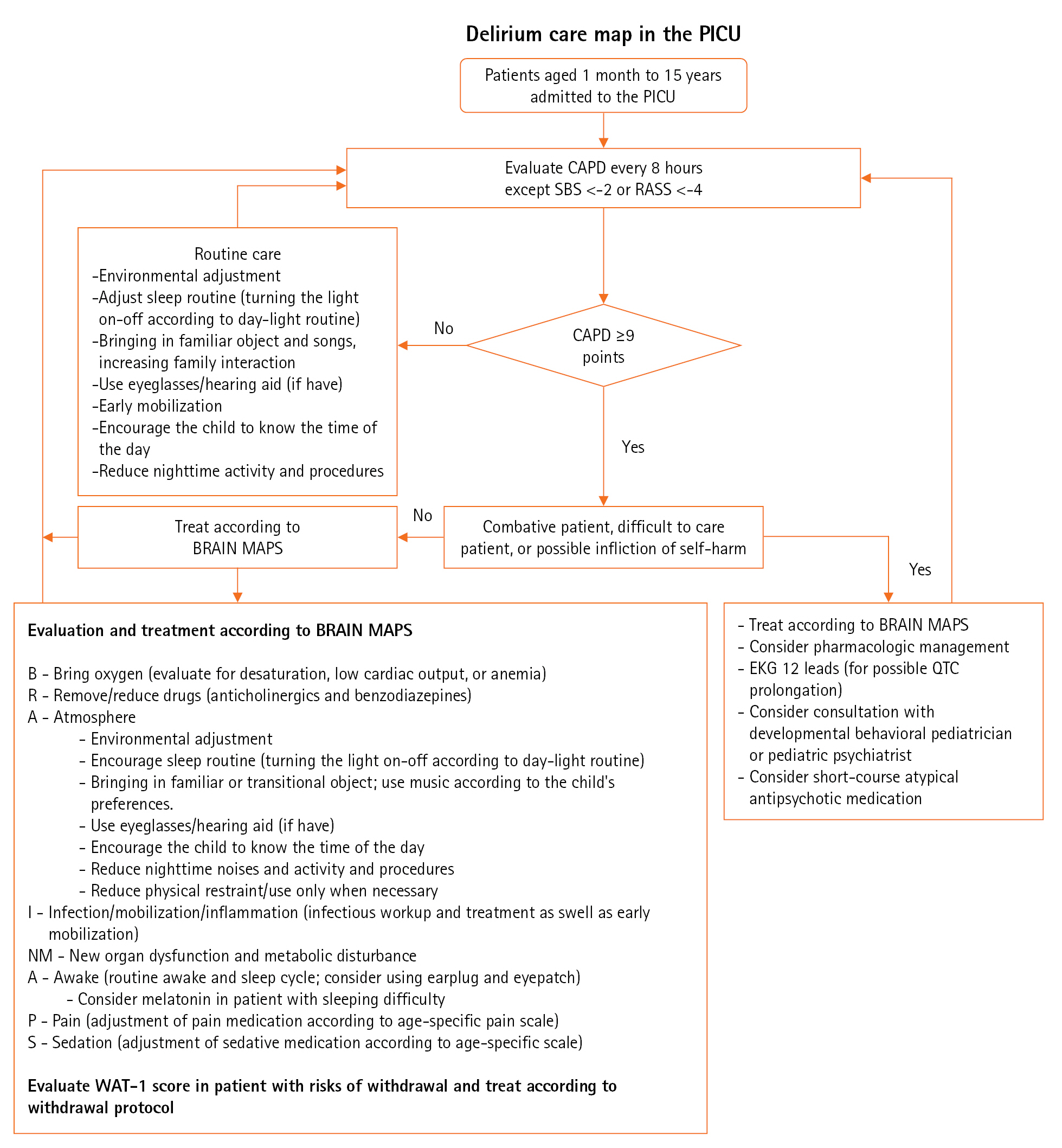
Figure 2. The flowchart for the study. All patients were followed until pediatric intensive care unit (PICU) discharge and were included for the final analysis.
Table 1.Demographic data among intervention groups
|
Factor |
All patients (n=120) |
Pre-intervention (n=58) |
Post-intervention (n=62) |
P-value |
|
Median age (yr) |
3.1 (0.9–6.8) |
3.1 (0.9–5.0) |
3.0 (0.6–7.8) |
0.985 |
|
Age <2 yr |
43 (35.8) |
20 (34.5) |
23 (37.1) |
0.850 |
|
Male |
68 (56.7) |
33 (56.9) |
35 (56.5) |
1.000 |
|
Underlying disease |
|
|
|
0.817 |
|
None |
23 (19.2) |
12 (20.7) |
11 (17.8) |
|
|
Cardiovascular |
50 (41.7) |
22 (37.9) |
28 (45.2) |
|
|
Neurologic |
9 (7.5) |
7 (12.1) |
2 (3.2) |
|
|
Genetic |
4 (3.3) |
2 (3.4) |
2 (3.2) |
|
|
Gastrointestinal |
7 (5.8) |
3 (5.2) |
4 (6.5) |
|
|
Hematology and oncology |
7 (5.8) |
3 (5.2) |
4 (6.5) |
|
|
Renal |
2 (1.7) |
1 (1.7) |
1 (1.6) |
|
|
Pulmonology |
5 (4.2) |
2 (3.4) |
3 (4.8) |
|
|
Trauma |
1 (0.8) |
1 (1.7) |
- |
|
|
Allergy |
7 (5.8) |
5 (8.7) |
2 (3.2) |
|
|
Endocrine |
2 (1.7) |
- |
2 (3.2) |
|
|
Rheumatology |
1 (0.8) |
- |
1 (1.6) |
|
|
Infectious disease |
2 (1.7) |
- |
2 (3.2) |
|
|
Admission diagnosis |
|
|
|
0.165 |
|
Cardiovascular |
46 (38.2) |
20 (34.6) |
26 (41.9) |
|
|
Gastrointestinal |
16 (13.3) |
8 (13.8) |
8 (12.9) |
|
|
Pulmonology |
15 (12.5) |
8 (13.8) |
7 (11.3) |
|
|
Neurologic |
12 (10.3) |
7 (12.1) |
5 (8.1) |
|
|
Asthmatic attack |
10 (8.3) |
9 (15.5) |
1 (1.6) |
|
|
Renal |
6 (5.0) |
2 (3.4) |
4 (6.5) |
|
|
Endocrine |
6 (5.0) |
1 (1.7) |
5 (8.1) |
|
|
Septic shock |
2 (1.7) |
2 (3.4) |
- |
|
|
Oncology |
2 (1.7) |
1 (1.7) |
1 (1.6) |
|
|
Trauma |
1 (0.8) |
- |
1 (1.6) |
|
|
Dental |
1 (0.8) |
- |
1 (1.6) |
|
|
Genetic |
1 (0.8) |
- |
1 (1.6) |
|
|
Orthopedics |
1 (0.8) |
- |
1 (1.6) |
|
|
Rheumatology |
1 (0.8) |
- |
1 (1.6) |
|
|
Delayed development |
37 (30.8) |
18 (31.0) |
19 (30.6) |
1.000 |
|
All-cause postoperative care |
56 (46.7) |
26 (44.8) |
30 (48.4) |
0.718 |
|
Median PRISM III |
2.0 (0.0–5.0) |
0.0 (0.0–5.0) |
2.0 (0.0–7.0) |
0.294 |
|
Median CAPD |
7.0 (3.0–12.0) |
7.0 (2.0–11.2) |
7.0 (3.0–12.0) |
0.874 |
|
Delirium |
50 (41.7) |
23 (39.7) |
27 (43.5) |
0.713 |
|
Delirium duration (hr) |
40.0 (24.0–74.0) |
56.0 (32.0–72.0) |
32.0 (18.0–80.0) |
0.274 |
|
Inotropes |
47 (39.2) |
21 (36.2) |
26 (41.9) |
0.577 |
|
Median vasoactive inotropic score [23] |
14.0 (7.0–24.0) |
16.0 (5.0–25.0) |
13.0 (7.8–21.4) |
0.192 |
|
ECMO support |
4 (3.3) |
1 (1.7) |
3 (4.8) |
0.619 |
|
Respiratory support |
|
|
|
0.453 |
|
None |
25 (20.8) |
9 (15.5) |
16 (25.8) |
|
Cannula |
23 (19.2) |
12 (20.7) |
11 (17.7) |
|
HFNC |
24 (20.0) |
14 (24.1) |
10 (16.1) |
|
MV |
48 (40.0) |
23 (39.7) |
25 (40.3) |
|
Median MV days |
2.5 (1.0–8.8) |
3.0 (1.0–9.0) |
2.0 (1.0–5.5) |
1.000 |
|
MV >7 days |
13/48 (27.1) |
9/23 (39.1) |
4/25 (16.0) |
0.106 |
|
Sedation |
42 (35.0) |
19 (32.8) |
23 (37.1) |
0.703 |
|
Sedation >7 days |
11/42 (26.2) |
7/19 (36.8) |
4/23 (17.4) |
0.180 |
|
NMB |
9 (7.5) |
4 (6.9) |
5 (8.1) |
1.000 |
|
Withdrawal |
5 (4.2) |
3 (5.2) |
2 (3.2) |
0.672 |
|
Median PICU days |
3.0 (1.0–8.0) |
3.0 (1.0–7.2) |
3.0 (1.0–8.0) |
0.436 |
|
PICU >7 days |
35 (29.2) |
16 (27.6) |
19 (30.6) |
0.841 |
|
Mortality |
3 (2.5) |
1 (1.7) |
2 (3.2) |
1.000 |
Table 2.Associating factors for positive delirium screening
|
Factor |
Delirium (n=50) |
No delirium (n=70) |
P-value |
|
Median age (yr) |
2.2 (0.8–6.5) |
3.6 (1.4–8.0) |
0.115 |
|
Age <2 yr |
24 (48.0) |
19 (27.1) |
0.022 |
|
Male |
29 (58.0) |
39 (55.7) |
0.853 |
|
Underlying disease |
|
|
0.819 |
|
None |
9 (18.0) |
14 (20.0) |
|
Cardiovascular |
28 (56.0) |
22 (31.5) |
|
Neurologic |
3 (6.0) |
6 (8.6) |
|
Genetic |
2 (4.0) |
2 (2.9) |
|
Gastrointestinal |
- |
7 (10.0) |
|
Hematology and oncology |
2 (4.0) |
5 (7.1) |
|
Renal |
1 (2.0) |
1 (1.4) |
|
Pulmonology |
2 (4.0) |
3 (4.3) |
|
Trauma |
- |
1 (1.4) |
|
Allergy |
2 (4.0) |
5 (7.1) |
|
Endocrine |
- |
2 (2.9) |
|
Rheumatology |
- |
1 (1.4) |
|
Infectious disease |
1 (2.0) |
1 (1.4) |
|
Admission diagnosis |
|
|
0.026 |
|
Cardiovascular |
28 (56.0) |
18 (25.8) |
|
|
Gastrointestinal |
2 (4.0) |
14 (20.0) |
|
|
Pulmonology |
8 (16.0) |
7 (10.0) |
|
|
Neurologic |
4 (8.0) |
8 (11.5) |
|
|
Asthmatic attack |
1 (2.0) |
9 (12.9) |
|
|
Renal |
2 (4.0) |
4 (5.7) |
|
|
Endocrine |
1 (2.0) |
5 (7.1) |
|
|
Septic shock |
1 (2.0) |
1 (1.4) |
|
|
Oncology |
1 (2.0) |
1 (1.4) |
|
|
Trauma |
1 (2.0) |
- |
|
|
Dental |
- |
1 (1.4) |
|
|
Genetic |
1 (2.0) |
- |
|
|
Orthopedics |
- |
1 (1.4) |
|
|
Rheumatology |
- |
1 (1.4) |
|
|
Delayed development |
26 (52.0) |
11 (15.7) |
<0.001 |
|
All-cause postoperative care |
23 (46.0) |
33 (47.1) |
1.000 |
|
PRISM III |
3.0 (0–7.2) |
0 (0–4.0) |
0.006 |
|
CAPD |
12.5 (11.0 -17.0) |
3.0 (0–5.2) |
<0.001 |
|
Inotropes |
29 (58.0) |
18 (25.7) |
0.001 |
|
Vasoactive inotropic score [23] |
14.0 (8.0–25.0) |
14.5 (5.0 -21.4) |
0.853 |
|
ECMO support |
4 (8.0) |
- |
0.028 |
|
Respiratory support |
|
|
<0.001 |
|
None |
6 (12.0) |
19 (27.1) |
|
Cannula |
4 (8.0) |
19 (27.1) |
|
HFNC |
7 (14.0) |
17 (24.3) |
|
MV |
33 (66.0) |
15 (21.5) |
|
MV duration |
5.0 (2.0–9.0) |
1.0 (1.0–1.5) |
0.013 |
|
MV >7 days |
12/33 (36.4) |
1/15 (6.7) |
0.040 |
|
Sedation |
29 (58.0) |
13 (18.6) |
<0.001 |
|
Sedation >7 days |
10/29 (34.5) |
1/13 (7.7) |
0.127 |
|
Neuromuscular blocker |
9 (18.0) |
- |
<0.001 |
|
Withdrawal |
5 (10.0) |
- |
0.011 |
|
Median PICU stay |
7.5 (3.0–13.0) |
2.0 (1.0–4.0) |
<0.001 |
|
PICU >7 days |
28 (56.0) |
7 (10.0) |
<0.001 |
|
Mortality |
1 (2.0) |
2 (2.7) |
1.000 |
Table 3.Univariate analyses for associating factors of positive delirium screening
|
Factor |
Crude odds ratio (95% CI) |
P-value |
|
Age <2 yr |
2.48 (1.15–5.32) |
0.022 |
|
Male |
1.10 (0.53–2.28) |
0.853 |
|
Presence of UD |
1.14 (0.45–2.88) |
0.819 |
|
All-cause postoperative care |
0.96 (0.46–1.98) |
1.000 |
|
Cardiovascular admission diagnosis |
3.61 (1.66–7.83) |
0.001 |
|
Delayed development |
5.81 (2.48–13.59) |
<0.001 |
|
Inotropic support |
3.99 (1.84–8.67) |
0.001 |
|
MV |
7.12 (3.14–16.12) |
<0.001 |
|
Prolonged MV >7 days |
8.00 (0.93–68.62) |
0.040 |
|
Sedation |
6.06 (2.66–13.80) |
<0.001 |
|
Prolonged sedation >7 days |
6.32 (0.72–55.81) |
0.127 |
|
Neuromuscular blocker |
32.28 (1.83–569.05) |
<0.001 |
|
Prolonged PICU stay >7 days |
11.46 (4.39–29.91) |
<0.001 |
Table 4.Multivariate analyses for associating factors of positive delirium screening
|
Factor |
Adjusted odds ratio (95% CI) |
P-value |
|
Age <2 yr |
3.41 (1.19–9.73) |
0.014 |
|
Cardiovascular admission diagnosis |
2.92 (0.78–10.91) |
0.112 |
|
Delayed development |
5.03 (1.73–14.59) |
0.004 |
|
Inotropic support |
0.26 (0.05–1.36) |
0.112 |
|
MV |
4.32 (0.47–40.12) |
0.197 |
|
Prolonged MV >7 daysa)
|
- |
- |
|
Sedation |
2.05 (0.18–23.75) |
0.565 |
|
Prolonged sedation >7 days |
- |
- |
|
Neuromuscular blockera)
|
- |
- |
|
Prolonged PICU stay >7 days |
11.58 (2.90–46.23) |
0.001 |
References
- 1. Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU. Crit Care Med 2014;42:656-63.PubMedPMC
- 2. Shieveld JN, Ista E, Knoester H, Molag ML. Pediatric delirium: a practical approach. In Rey JM, editor. IACAPAP e-textbook of child and adolescent mental health. International Association for Child and Adolescent Psychiatry and Allied Professions; 2015. p. 1-16.
- 3. World Health Organization. International statistical classification of diseases and related health problems 10th revision (ICD-10) [Internet]. World Health Organization. 2015;[cited 2023 Aug 20]. Available from: https://icd.who.int/browse10/2015/en#/F00-F09.
- 4. Ista E, van Beusekom B, van Rosmalen J, Kneyber MC, Lemson J, Brouwers A, et al. Validation of the SOS-PD scale for assessment of pediatric delirium: a multicenter study. Crit Care 2018;22:309. ArticlePubMedPMCPDF
- 5. Silver G, Traube C, Gerber LM, Sun X, Kearney J, Patel A, et al. Pediatric delirium and associated risk factors: a single-center prospective observational study. Pediatr Crit Care Med 2015;16:303-9.PubMedPMC
- 6. Silver G, Traube C, Kearney J, Kelly D, Yoon MJ, Nash Moyal W, et al. Detecting pediatric delirium: development of a rapid observational assessment tool. Intensive Care Med 2012;38:1025-31.ArticlePubMedPDF
- 7. Traube C, Silver G, Reeder RW, Doyle H, Hegel E, Wolfe HA, et al. Delirium in critically ill children: an international point prevalence study. Crit Care Med 2017;45:584-90.ArticlePubMedPMC
- 8. Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, et al. Diagnosing delirium in critically ill children: validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med 2011;39:150-7.ArticlePubMedPMC
- 9. Smith HA, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Savage S, et al. The preschool confusion assessment method for the ICU: valid and reliable delirium monitoring for critically ill infants and children. Crit Care Med 2016;44:592-600.PubMedPMC
- 10. Traube C, Silver G, Gerber LM, Kaur S, Mauer EA, Kerson A, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med 2017;45:891-8.
- 11. Leentjens AF, Schieveld JN, Leonard M, Lousberg R, Verhey FR, Meagher DJ. A comparison of the phenomenology of pediatric, adult, and geriatric delirium. J Psychosom Res 2008;64:219-23.ArticlePubMed
- 12. Tasker RC, Menon DK. Critical care and the brain. JAMA 2016;315:749-50.ArticlePubMed
- 13. Turkel SB. Pediatric delirium: recognition, management, and outcome. Curr Psychiatry Rep 2017;19:101. ArticlePubMedPDF
- 14. Patel AK, Bell MJ, Traube C. Delirium in pediatric critical care. Pediatr Clin North Am 2017;64:1117-32.ArticlePubMed
- 15. Jesus AO, Jones L, Linares R, Buck ML, Frank DU. Management of hyperactive delirium in the pediatric intensive care unit: case series of three young children. J Pediatr Intensive Care 2020;9:119-23.ArticlePubMed
- 16. Smith HA, Berutti T, Brink E, Strohler B, Fuchs DC, Ely EW, et al. Pediatric critical care perceptions on analgesia, sedation, and delirium. Semin Respir Crit Care Med 2013;34:244-61.ArticlePubMed
- 17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse 2017;37:e9-18.ArticlePubMedPDF
- 18. Smith HA, Besunder JB, Betters KA, Johnson PN, Srinivasan V, Stormorken A, et al. 2022 Society of Critical Care Medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med 2022;23:e74-110.ArticlePubMed
- 19. Kerson AG, DeMaria R, Mauer E, Joyce C, Gerber LM, Greenwald BM, et al. Validity of the Richmond Agitation-Sedation Scale (RASS) in critically ill children. J Intensive Care 2016;4:65. ArticlePubMedPMCPDF
- 20. Curley MA, Harris SK, Fraser KA, Johnson RA, Arnold JH. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med 2006;7:107-14.ArticlePubMedPMC
- 21. Hagan JF, Shaw JS, Duncan PM. Bright futures: guidelines for health supervision of infants, children, and adolescents. 3rd ed. American Academy of Pediatrics. 2008.
- 22. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996;24:743-52.PubMed
- 23. Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth 2021;35:3067-77.ArticlePubMed
- 24. Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019;47:3-14.ArticlePubMedPMC
- 25. Michel J, Schepan E, Hofbeck M, Engel J, Simma A, Neunhoeffer F. Implementation of a delirium bundle for pediatric intensive care patients. Front Pediatr 2022;10:826259. ArticlePubMedPMC
- 26. Franken A, Sebbens D, Mensik J. Pediatric delirium: early identification of barriers to optimize success of screening and prevention. J Pediatr Health Care 2019;33:228-33.ArticlePubMed
- 27. Simone S, Edwards S, Lardieri A, Walker LK, Graciano AL, Kishk OA, et al. Implementation of an ICU bundle: an interprofessional quality improvement project to enhance delirium management and monitor delirium prevalence in a single PICU. Pediatr Crit Care Med 2017;18:531-40.ArticlePubMed
- 28. Meyburg J, Dill ML, Traube C, Silver G, von Haken R. Patterns of postoperative delirium in children. Pediatr Crit Care Med 2017;18:128-33.ArticlePubMed
- 29. Semple D, Howlett MM, Strawbridge JD, Breatnach CV, Hayden JC. A systematic review and pooled prevalence of delirium in critically ill children. Crit Care Med 2022;50:317-28.ArticlePubMed
Citations
Citations to this article as recorded by

 , Thananya Thadahirunchot2
, Thananya Thadahirunchot2






 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite