Abstract
-
Background
- Coronavirus disease 2019 (COVID-19) pandemic disrupted adherences to healthcare-associated infection (HAI) prevention protocols. Herein, we studied the characteristics of all HAIs occurring in critically ill COVID-19 patients.
-
Methods
- A retrospective, single-center cohort of critical COVID-19 patients during 2021. Microbiological samples were collected if HAI was suspected. We analyzed all factors that could potentially induce HAI, using septic shock and mortality as endpoints.
-
Results
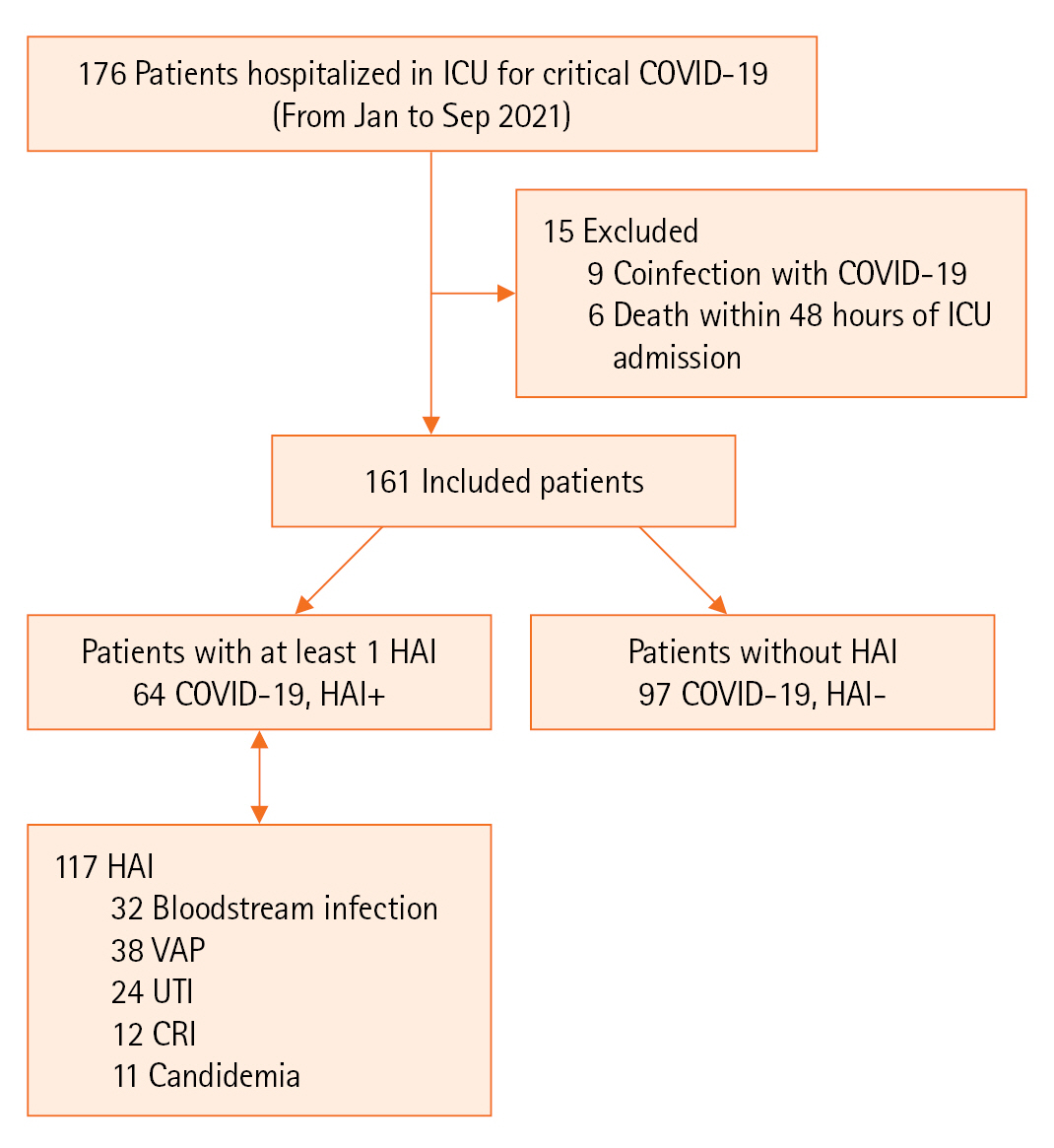
- Sixty-four among 161 included patients (39.7%) presented a total of 117 HAIs with an incidence density of 69.2 per 1,000 hospitalization days. Compared to the prior COVID-19 period (2013–2019), the identification of HAI increased in 2021. HAIs were classified into ventilator-associated pneumonia (VAP; n=38), bloodstream infection (n=32), urinary tract infection (n=24), catheter-related infection (n=12), and fungal infection (n=11). All HAIs occurred significantly earlier in the post–COVID-19 period (VAP: 6 vs. 10 days, P=0.045, in 2017 and 2021). Acinetobacter baumannii (39.5%) and Klebsiella pneumoniae (27%) were the most commonly isolated pathogens that exhibited a multidrug-resistant (MDR) profile, observed in 89% and 64.5%, respectively. The HAI factors were laboratory abnormalities (odds ratio [OR], 6.4; 95% confidence interval [CI], 2.3–26.0), cumulative steroid dose (OR, 1.9; 95% CI, 1.3–4.0), and invasive procedures (OR, 20.7; 95% CI, 5.3–64.0). HAI was an independent factor of mortality (OR, 8.5; P=0.004).
-
Conclusions
- During the COVID-19 era, the incidence of HAIs increased and MDR isolates remained frequent. A severe biological inflammatory syndrome, invasive devices, and elevated cumulative steroid dosages were related to HAIs. HAI was a significant death factor.
-
Keywords: COVID-19; critical care; epidemiology; healthcare-associated infections; prognosis
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an infectious respiratory disease caused by the novel corona virus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The COVID-19 pandemic disrupted healthcare systems around the world [1,2]. The large number of critically ill patients necessitated the rapid expansion of intensive care unit (ICU) bed capacity, which is commonly called as expanded ICU. In addition, there was a request from non-ICU units to strengthen human and logistical resources.
As a result, it was not easy to properly adhere to the guidelines for the prevention of healthcare-associated infections (HAIs). Several studies reported that the proportion of HAIs during the pandemic was higher compared to the period before the pandemic [3,4]. Indeed, patients with COVID-19 who required intensive care were more vulnerable to HAIs due to the use of invasive mechanical ventilation, co morbidities, immune suppression induced by both the SARS-CoV-2 infection and the severe disease itself, the use of immunomodulators (e.g., steroids) and other invasive life-sustaining procedures [5,6]. These factors inevitably had a detrimental impact on patient outcomes.
In this study, we sought to determine the incidence rates, the causative microorganisms with antimicrobial resistance profiles, the risk factors, and the impact of HAIs diagnosed in ICU patients hospitalized for critical COVID-19. Additionally, we compared the epidemiology of HAIs in the era of COVID-19 versus that before the onset of the pandemic.
MATERIALS AND METHODS
Study Design and Ethical Status
This retrospective, single-center study was conducted in the medical ICU of a tertiary teaching hospital over a 9-month period (from January 2021 to September 2021). During this period, this facility experienced three significant peaks (January 2021, April 2021, and June/July 2021) of COVID-19 infections. Additionally, the ICU underwent logistical modifications to receive only critically ill COVID-19 patients.
The study was approved by the local Ethics Committee of La Rabta Hospital (No. 2021-I). Given the retrospective nature of this study, the need for written informed consent was waived.
Study Population
All eligible patients were 18yearsor older, were diagnosed with COVID-19 and required intensive care. SARS-CoV-2 infection was confirmed by a positive reverse transcription polymerase chain reaction test [7] from a nasopharyngeal swab. The patients who were discharged or who died within 48 hours of admission and those who had an infection that was not associated with healthcare were excluded from this study.
Main Endpoint
First, we determined the incidence rate of HAIs (at all locations combined and for each kind of HAI), described the microbiological characteristics, and compared the current epidemiology to that in the pre–COVID-19 era. Second, we studied the risk factors contributing to the occurrence of HAIs. Third, we assessed the impact of the HAI on the outcome parameters (namely septic shock, ventilator-free days, length of stay, and mortality). The comparison of the HAI epidemiology between the post–COVID-19 period versus the pre–COVID-19 period was performed based on the results of a study conducted in 2013 and published in 2017 [8] and to the unpublished local data of 2018 and 2019.
Microbiological Sampling Policy
In the presence of clinical or biological suspicion of HAI, the following microbiological cultures were prepared: a cytobacteriological examination of sputum was performed in patients with spontaneous breathing or via the tracheal aspiration (TA) of ventilated patients. For all patients with a suspected HAI, blood cultures were used to determine an aerobic or anaerobic environment, in addition to a cytobacteriological examination of urine (CBEU). Simultaneously, a fungal investigation was performed, including a blood culture on Sabouraud's medium and a colonization index on five sites (buccal, nasal, rectal, axillary, and inguinal).
Microbiologic Methods for Organism Identification
The Vitek 2 automated system (bioMérieux) was used for isolate identification and antimicrobial susceptibility testing. Minimum inhibitory concentrations were established according to the European Committee on Antimicrobial Susceptibility Testing breakpoints. For the identification of Candida albicans, the chlamydosporulation test on AT (Agar, Tween) or PCB (Potato, Carrot, Bile) medium was used. For non-albicans species, identification was based on the morphological appearance on AT or PCB media and on the Auxacolor sugar assimilation gallery (Bio-Rad).
Definitions
Infections were considered health care associated if they occurred within a minimum of 48 hours following admission to the ICU. The following HAIs were diagnosed: ventilator-associated pneumonia (VAP), hospital-acquired pneumonia, urinary tract infection (UTI), bloodstream infection (BI), catheter-related infection (CRI), and suspected or proven invasive candidiasis. A documented bacterial infection was defined as the presence of a bacterium at a significant concentration (>106 in TA for VAP, >103 in the catheter culture for CRI, and >105 in the CBEU for UTI) and responsible for clinical or biological signs of sepsis.
Multidrug-resistant (MDR) bacteria were defined as all microorganisms resistant to at least one agent among three or more antimicrobial classes [9] and microorganisms known to have specific mechanisms of antibiotic resistance, such as methicillin-resistant Staphylococcus aureus (MRSA).
Collected Data
For each patient admitted for COVID-19, with an ICU-stay longer than 48 hours, the following information was collected and recorded in an electronic database: basic characteristics (age, sex, body mass index, ICU stay during the year prior to the current hospital admission, history of infection treated with antimicrobials during the year previous to admission, origin and length of stay before ICU admission, co morbidities, severity scores, biological data, use of invasive procedures, bacteriological and fungal results, and outcome data. Among the factors related to HAI, we studied the steroid cumulative dose that corresponded to dexamethasone at a dose of 6 mg/day for the entire duration of treatment.
To note that in the COVID-19, HAI+ group, outcome parameters of shock, pulmonary embolism, and other complications were considered when they occurred after HAI onset. This approach was used to avoid biasing the cause-and-effect link (HAI/complication) due to chronological factors.
Statistical Analyses
Descriptive quantitative variables were expressed as mean and standard deviation or median and interquartile range, according to the distribution. Categorical variables were reported as numbers and percentages. The groups (with HAI versus without HAI) were compared using parametric or non-parametric tests, according to the distribution of the data. The time scale of analysis was the period from the time of ICU admission until the date of discharge from the ICU or death. The HAI incidence rate was calculated as the number of HAI episodes per 1,000 days of ICU hospitalization for all included patients. The VAP incidence rate was calculated as the total number of VAP episodes during the study period divided by 1,000 days of ventilation for all patients included. The UTI incidence rate was calculated as the total number of UTI episodes during the study period divided by 1,000 days of bladder catheterization for all patients included. Comparisons of means (such as the time to onset of the different HAIs) were performed using the non-parametric Kruskal-Wallis test.
Risk factors for HAIs and those of mortality were both explored using logistic regression modeling. This multivariate analysis method involved factors with a P-value less than0.05 in the univariate analysis (COVID-19 patients with HAIs versus those without HAIs for the first analysis and between COVID-19 survivors vs. COVID-19 no survivors for the second). Each measure was expressed as an odds ratio (OR) with the corresponding 95% confidence interval (CI). All statistical tests were two-sided, and P<0.05 was selected to indicate statistical significance. Statistical analysis was performed using IBM SPSS ver.20 software (IBM Corp.).
RESULTS
During the study period, 161 patients were included in the analysis. Among them, 64 (39.7%) presented 117 HAIs, resulting in an incidence density of 69.2 per 1,000 of hospitalization days, as illustrated in Figure 1.
Clinical Characteristics
The included patients had an average age of 58 years and were predominately male. The most frequently reported co morbidities were hypertension and diabetes. Nearly three-quarters of the patients had received antibiotics in the 3 months prior to admission, and 52% of the patients required invasive ventilation. Regarding steroids, for all infected patients, dexamethasone was administered at a dosage of 6 mg/day, but the duration of this regimen differed between patients. Thus, we present this result as a cumulative dose (mg per day). All clinical data (baseline and during follow-up) are provided in Table 1.
HAI Epidemiology
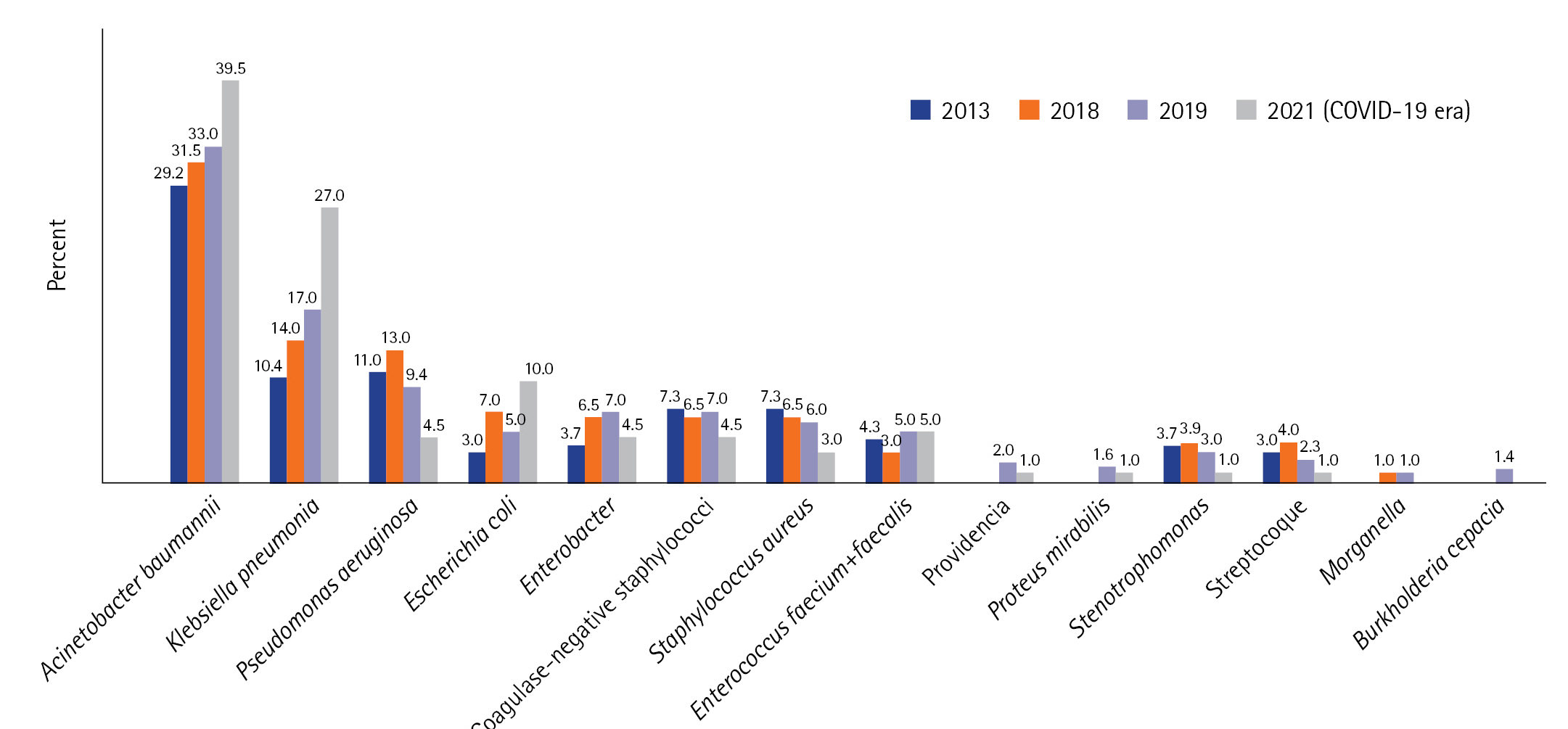
The most frequent HAI was due to VAP, accounting for 32.5% (n=38/117) of cases, occurring at a median time of 6 days after admission [3-9]. The second most frequent HAI was BI (n=32/117, 27.5%), which occurred at a median time of 7 days [4-12]. The predominant isolates in all HAIs were Acinetobacter baumannii and Klebsiella pneumoniae (39.5% and 27% of cases, respectively). A. baumannii had a profile of sensitivity to colistin in 89% of cases, and K. pneumoniae had an extended-spectrum beta-lactamase antibiotic-resistant profile in 64.5% of cases and carbapenem resistance in 26% of cases. Proportions of MDR among the other isolated species were Pseudomonas aeruginosa (45%), Enterobacteriaceae species other than K. pneumoniae (39%), Staphylococcus aureus (44%), and Enterococci (26%).
For fungal infections, 11 cases of candidemia were recorded, and Candida albicans was the exclusive isolate. A colonization index assessment was performed in 88 patients and was repeated two to three times (weekly) for 36 patients who stayed longer than 1 week. A total of 144 colonization indexes was obtained, of which 82 (57%) were positive, 48 were poorly colonized (colonization index <0.5), and 34 were ≥ 0.5. The most colonized sites were oral (n=60), anal (n=33), and nasal (n=29). Candida albicans was most frequently isolated (54%), followed by Candida glabrata (23%).
Compared to the epidemiology before COVID-19 (in 2013, 2018, and 2019), there was an increase in the incidence density of HAIs, particularly regarding VAP and bacteremia. Conversely, the incidence of CRI decreased from 2013 to 2021 (Figure 2). The time to onset for all types of HAI was shorter in the COVID-19 period (Figure 2). The distribution of microorganisms involved in infections was similar between the two periods (before and during the COVID-19 era). However, during the COVID-19 period, we observed larger proportions of A. baumannii and K. pneumoniae, decreases of P. Aeruginosa and Staphylococci, and an increase of Escherichia coli (Figure 3).
HAI Risk Factors
Univariate analysis identified 13 variables as significant with P<0.05: age, hypertension, immunosuppression, hyperglycemia, elevated C-reactive protein (CRP), lymphopenia, reduced ratio of arterial blood (PaO2) to fraction of inhaled oxygen (FiO2) (P/F ratio), computed tomography (CT) lesions >50%, invasive ventilation, cumulative steroid dose, venous catheter, arterial catheter, and bladder catheterization (Table 2).
Given the limited number of patients (n=161) and the large number of variables to be included in the multivariate analysis (13 variables), we grouped certain variables into categories. The resulting six categories were as follows: age, comorbidities (hypertension and immunosuppression), laboratory abnormalities (hyperglycemia, elevated CRP, lymphopenia, reduced P/F), CT lesions > 50%, cumulative steroid dose, and invasive procedures (invasive ventilation, venous catheter, arterial catheter, and bladder catheterization). The factors associated with HAIs in COVID-19 patients in an ICU were biological abnormalities (OR, 6.4; 95% CI, 2.3–26.0), cumulative steroid dose > 60 mg per day (OR, 1.9; 95% CI, 1.3–4.0), and invasive procedures (OR, 20.7; 95% CI, 5.3–64.0).
Impact on Outcome
Patients who exhibited HAIs showed more frequent septic shock, greater numbers of ventilator days and ICU days, and higher mortality (Table 3). The analysis of mortality found that HAI was an independent factor of death in critical COVID-19 patients with an OR of 8.49 and a 95% CI of 2.56–32.00. Other poor prognosis cofactors were observed, including stage 3 acute respiratory distress syndrome, invasive ventilation, and septic shock, as shown in Table 4.
DISCUSSION
Compared to the pre–COVID-19 era, this study demonstrated a notable increase in HAIs in critical COVID-19 patients. Specifically, there were significant increases in VAP, UTIs, and BIs. Additionally, all types of HAIs had shorter onset during the COVID-19 period. A. baumannii and K. pneumoniae were the most commonly identified microorganisms. Invasive devices, biological disorders, and cumulative steroid dose were the independent factors of HAIs. COVID-19 patients presenting HAIs showed a higher incidence of septic shock and required greater ventilator days and ICU days. Mortality was significantly higher and HAI was an independent factor associated with death (OR, 8.49; 95% CI, 2.56–32.00).
HAIs represent one of the most common adverse events in healthcare establishments [9-13]. At the onset of the COVID-19 pandemic, there was a reduction in HAIs, perhaps due to the strengthening of hygiene measures, mainly the use of hydroalcoholic gels [14].However, the massive influx of critical cases and the rapid reorganization of ICUs reduced the focus on traditional measures that reduce HAIs. We mentioned above that our unit underwent logistical and functional change during the pandemic. These changes briefly consisted of a rapid increase in ICU beds and the recruitment of caregivers from non-ICU services (all logistical and functional changes are presented in the Supplementary Material 1). We suggest that these changes might affect HAI models and contribute to the increase in HAI incidence. Moreover, the predominance of Acinetobacter and Klebsiella may represent an indirect witness to the failure of hygiene rules. In fact, their transmission essentially occurs via handling, inert surfaces, and invasive ventilation equipment. Furthermore, the higher density of patients likely contributed to an increase in the spread of these bacteria.
The increase in HAI incidence in COVID-19 patients has been previously described [15-17].The percentage of HAI among all patients in our series (39.7%) was similar to that of Somers et al. [17] in ventilated patients (40%). VAP occurred in 32.5% of our population, which was consistent with several previous results: 32.3% in a Chinese report [18] and 38% in a Spanish study [19]. A higher value was reported by an Italian study at 50% [20]. We explained the increase in VAP during COVID-19 by the respiratory tropism of the virus and the frequent use of ventilation, sedation, and neuromuscular blocking agents [21,22].
Our rate of BI (27.5%) was similar to that reported by Grasselli et al. (23.6%) [20] but was higher than that of a French study (14.9%) [23]. For UTIs, we showed an incidence of 22.2%. This was significantly higher than those reported by Grasselli et al. [20]: 7.7% [20], Falcone et al. [24]: 9.8%, and Bardi et al. [25]: 5%. Our incidence of CRI at 10.25% was close to that reported in the Italian study cited above (9.5%) [20], higher than that of Falcone et al. (6.6%) [24], and lower than that of Bardi et al. (20%) [25]. The changes and variability in the application of hygiene protocols may represent the main factor in explaining these gaps in incidences between countries. Nevertheless, these changes should be interpreted according to the COVID-19 situation (e.g., a surge in patients, medical supplement status), length of stay and exposure to invasive devices, the quality of programs for the prevention of HAIs, among other factors.
Unlike what we found for microorganisms, where Gram-negative bacilli predominated, the most commonly identified were S. aureus, both methicillin-susceptible and methicillin-resistant S. aureus followed by Pseudomonas in other reports [16,17,26]. Invasive procedures were strongly associated with HAIs. In fact, a patient is often infected by their own germs during invasive care (surgical procedures, invasive ventilation, vascular catheterization, urinary catheterization, etc.), and caregivers act as a vector of transmission. Otherwise, the association between CRP and HAI was observed by Falcone et al. [24], where a CRP on admission > 7mg/dl increased the risk of HAI with an OR of 3.59 and a 95% CI of 1.7–7.7 (P=0.001). High white blood cell and procalcitonin levels were associated with HAI with respective OR of 8.38 (95% CI, 1.07–65.55; P=0.04) and 4.92 (95% CI, 1.39–17.33; P=0.013)[27]. Hyperglycemia may be a consequence of a systemic inflammatory response and may serve as a marker of immunocompetence [28,29]. Consistent with our result, some reports found that hyperglycemia represents a risk factor for HAI [28,29].
The other factor we identified as influential was steroids. The latter exerts an inhibitory effect on the acquired and innate immune system. Therefore, this increases the risk of infection depending on the dose and time. Steroids should be used in a targeted manner, particularly in the context of infectious pathologies. Several studies reported that mortality increased when HAI occurred in critically ill patients [16,18,19,25,30]. Patients with HAI had longer ventilation days (OR, 3.31; 95% CI, 1.67–6.56; P=0.001), longer ICU stays (OR, 1.90; 95% CI, 1.06–3.40; P=0.03), and a higher 60-day mortality (OR, 1.86; 95% CI, 1.05–3.29; P=0.03) in a large multicenter study [16]. Mortality was twice as likely when HAI was complicated by septic shock, whereas uncomplicated infections did not affect mortality [20]. Septic shock was also a factor of mortality in our series (OR, 9.8; 95% CI, 4.0–38.7).
Our study reports original results focused on the particular context of the COVID-19 outbreak. This work enriches our national registry on HAIs. The comparison with previous epidemiology adds a point of strength. However, this work has certain limitations: first, the retrospective and single-center design could influence the validity of our conclusions. Second, the small sample size could affect the multivariate analysis because we grouped several variables into categories to compensate for the small sample size. Third, the lack of cost estimates regarding HAIs may be considered a limitation.
We concluded that the rate of HAIs increased from the pre–COVID-19 period to the COVID-19 pandemic period and this was mostly related to increased VAP and UTI complications. MDR isolates continued to be the pathogens most frequently responsible for these infections. The most highly related factors were severe biological inflammatory syndrome, invasive devices, and elevated cumulative steroid dose. We found that an HAI amplified the risk of death by a factor of eight. These findings indicate the need to develop a continuous surveillance system to identify and fight HAIs and strengthen procedures in the event of a pandemic.
KEY MESSAGES
▪ Compared to the pre-coronavirus disease 2019 era, ventilator-associated pneumonia, bloodstream infections, and urinary tract infections increased, while catheter-related infections decreased.
▪ The use of invasive devices, the presence of medical disorders, and higher cumulative steroid doses were independently related to healthcare-associated infections in COVID-19 patients.
▪ The risk of mortality among COVID-19 patients presenting HAIs increased by a factor of eight compared to those without any HAIs.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: AT. Methodology: AT. Formal analysis: BT. Data curation: SS, LM, ES. Visualization: SS, AM, LM, ES, BT. Writing–original draft: AT. Writing–review & editing: SA.
Acknowledgments
The abstract of this paper was presented as Flash-Com (FC 135, title: Healthcare-Related Infections in Critical Patients with COVID-19: Epidemiology, Risk Factors and Outcomes) at the Congress of the French Intensive Care Society (FICS) on June 23, 2022, in Paris (Palais de Congrès de Paris, Porte Maillot), France.
All the authors express their gratitude to Mr. Moez Ghrairi, English faculty member, for his help in proofreading this research paper.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2023.00773.
Figure 1.Study diagram. ICU: intensive care unit; COVID-19: coronavirus disease 2019; HAI: healthcare-associated infection; VAP: ventilator-associated pneumonia; UTI: urinary tract infection; CRI: catheter-related infection.
Figure 2.Incidence density (A) and time to onset (B) of healthcare-associated infections (HAIs) before and during the coronavirus disease 2019 (COVID-19) era. 2013 results from [8]; 2018 and 2019 results: not published data. HD: hospitalization day; VAP: ventilator-associated pneumonia; VD: ventilator day; CRI: catheter-related infection; CD: catheter day; UTI: urinary tract infection; BCD: bladder catheterization day; FI: fungal infection.
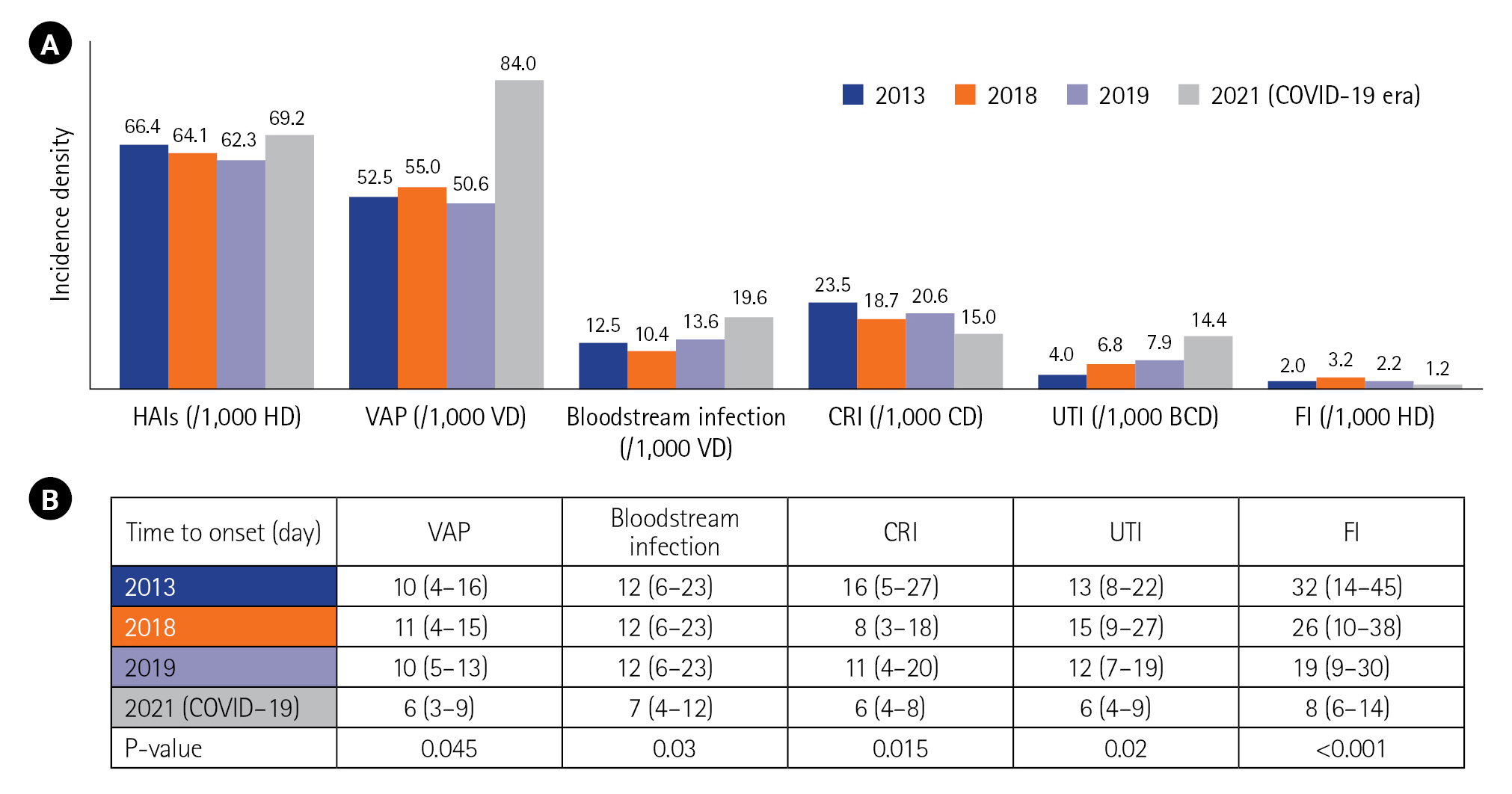
Figure 3.Distribution of microorganisms before and during corona virus disease 2019 (COVID-19) in percent (%). 2013 results from [8]; 2018 and 2019 results: not published data.

Table 1.Clinical characteristics
|
Variable |
Value(n=161) |
|
Age (yr) |
58 (48–69) |
|
Male: female |
95:66 |
|
Origin |
|
|
Emergency room |
95 (59) |
|
LOS before ICU (day) |
3 (2–5) |
|
Comorbidity |
|
|
Hypertension |
61 (38) |
|
Diabetes mellitus |
55 (34) |
|
Cardiac failure |
12 (7.5) |
|
Dyslipidemia |
33 (20.5) |
|
Chronic respiratory failure |
7 (4.5) |
|
Chronic renal failure |
15 (9.5) |
|
Severity score |
|
|
SAPS II |
30 (22–48) |
|
APACHE II |
16 (10–24) |
|
SOFA |
4 (3–4) |
|
Hospitalization in a care structure the previous year |
11 (7) |
|
Antibiotics in the previous 3 months |
117 (73) |
|
CT scan lesions >50% |
105 (65) |
|
Invasive ventilation |
84 (52) |
|
Invasive device |
|
|
Venous catheter |
88 (55) |
|
Arterial catheter |
46 (28.5) |
|
Bladder catheterization |
91 (57) |
|
Antibiotic duration (day) |
4 (2–8) |
|
Steroid cumulative days (mg per day) |
66 (48–90) |
|
ECMO |
2 |
Table 2.Comparison of all variables according to the occurrence of HAI (univariate analysis)
|
Variable |
COVID-19, HAI+ (n=64) |
COVID-19, HAI– (n=97) |
P-value |
|
Clinical variable |
|
|
Age (yr) |
60 (52–71) |
55 (44–63) |
0.02 |
|
Male:female |
36:28 |
59:38 |
0.62 |
|
ER origin |
38 (60) |
57 (59) |
1.00 |
|
Stay before ICU (day) |
3 (1–5) |
3 (2–6) |
0.06 |
|
Comorbidity |
|
|
|
|
Hypertension |
34 (53) |
27 (28) |
0.002 |
|
Diabetes |
27 (42) |
28 (29) |
0.09 |
|
Heart failure |
6 (9) |
6 (6) |
0.54 |
|
Dyslipidemia |
11 (17) |
22 (23) |
0.43 |
|
Chronic respiratory failure |
5 (8) |
3 (3) |
0.15 |
|
Chronic renal failure |
8 (13) |
7 (7) |
0.27 |
|
Immunosuppression |
6 (9) |
2 (2) |
0.05 |
|
SOFA score |
4 (3–4) |
4 (3–4) |
0.18 |
|
Antimicrobials in the previous 3 months |
43 (67) |
74 (76) |
0.21 |
|
Laboratory and CT variable |
|
|
|
|
Hyperglycemia on admission |
48 (75) |
36 (37) |
<0.001 |
|
D-dimers (µg/L) |
1,305 (677–2,640) |
1,086 (651–1,777) |
0.19 |
|
CRP (mg/L) |
134 (75–238) |
104 (48–170) |
0.02 |
|
WBC (×103/ml) |
9.2 (6.6–13.9) |
8.4 (6.4–11.8) |
0.12 |
|
Lymphocytes (elements/ml) |
590 (442–860) |
750 (540–1090) |
0.01 |
|
P/F ratio |
75.5(63–91) |
92 (67–131) |
0.02 |
|
CT scan lesions> 50% |
51 (80) |
54 (56) |
0.01 |
|
Therapeutic variable |
|
|
|
|
Invasive ventilation |
56 (88) |
28 (29) |
<0.001 |
|
Antimicrobial duration (day) |
4 (3–7) |
4 (2–6) |
0.70 |
|
Steroid cumulative dose (mg/day) |
72 (54–102) |
60(42–72) |
0.001 |
|
ECMO |
1 |
1 |
1.00 |
|
Venous catheter |
58 (90) |
30 (31) |
<0.001 |
|
Femoral |
57 |
31 |
<0.001 |
|
Under keyboard |
4 |
1 |
0.08 |
|
Chinstrap |
17 |
3 |
<0.001 |
|
Arterial catheter |
36 (56) |
10 (11) |
<0.001 |
|
Femoral |
4 |
1 |
0.07 |
|
Radial |
33 |
8 |
<0.001 |
|
Bladder catheterization |
61 (96) |
30 (31) |
<0.001 |
Table 3.Impact of HAIs on outcome
|
Outcome parameter |
COVID-19, HAI+ (n=64) |
COVID-19, HAI– (n=97) |
P-value |
|
Shock |
45 (70.0) |
18 (18.5) |
<0.001 |
|
Septic |
41 (64.0) |
13 (13.4) |
<0.001 |
|
Cardiogenic |
1 (1.5) |
5 |
0.020 |
|
Mixed |
3 (4.6) |
0 |
- |
|
Pulmonary embolism |
7 (11.0) |
4 (4.0) |
0.110 |
|
Other complication |
4 (6.3) |
2 (2.0) |
0.009 |
|
Myocarditis |
0 |
1 |
- |
|
Arrhythmia |
2 |
0 |
- |
|
Coronary insufficiency |
1 |
1 |
- |
|
Vein thrombosis |
1 |
0 |
- |
|
Ventilator day |
6 (3–0) |
2 (1–4) |
<0.001 |
|
ICU LOS |
12 (5–19) |
8 (3–13) |
0.002 |
|
Mortality |
54 (84.3) |
33 (34.0) |
<0.001 |
Table 4.Factors related to mortality
|
Variable |
Survivor (n=74) |
Non survivor (n=87) |
P-value |
Multivariable analysis, OR (95% CI) |
|
HAI |
10 (13.5) |
54 (62.0) |
<0.001 |
8.5(2.6–32.0) (P=0.004) |
|
Male:female (ratio) |
46:28 (1.64) |
49:38 (1.28) |
0.520 |
- |
|
Age (yr) |
56 (49–67) |
61 (53–71) |
0.006 |
NS |
|
SOFA score |
3.5 (2–4) |
4.0 (3–4) |
0.040 |
NS |
|
P/F ratio |
96 (75–135) |
73 (61–90) |
<0.001 |
NS |
|
Stage 3 ARDS |
33 (44.6) |
72 (83.0) |
<0.001 |
7.2(2.1–26.6) (P=0.007) |
|
Invasive ventilation |
5 (6.7) |
79 (91.0) |
<0.001 |
23.5 (9.9–84.0) (P<0.001) |
|
Septic shock |
5 (6.7) |
49 (56.5) |
<0.001 |
9.8 (4.0–38.7) (P=0.002) |
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.ArticlePubMedPMC
- 2. Conway Morris A, Kohler K, De Corte T, Ercole A, De Grooth HJ, Elbers PW, et al. Co-infection and ICU-acquired infection in COIVD-19 ICU patients: a secondary analysis of the UNITE-COVID data set. Crit Care 2022;26:236. PubMedPMC
- 3. Despotovic A, Milosevic B, Milosevic I, Mitrovic N, Cirkovic A, Jovanovic S, et al. Hospital-acquired infections in the adult intensive care unit-Epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am J Infect Control 2020;48:1211-5.ArticlePubMed
- 4. Barrasa H, Martín A, Maynar J, Rello J, Fernández-Torres M, Aguirre-Quiñonero A, et al. High rate of infections during ICU admission of patients with severe SARS-CoV-2 pneumonia: a matter of time? J Infect 2021;82:186-230.Article
- 5. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 2020;81:266-75.ArticlePubMedPMC
- 6. Contou D, Claudinon A, Pajot O, Micaëlo M, Longuet Flandre P, Dubert M, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care 2020;10:119. ArticlePubMedPMCPDF
- 7. Hanson KE, Caliendo AM, Arias CA, Englund JA, Hayden MK, Lee MJ, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of Coronavirus Disease 2019 (COVID-19): serologic testing. Clin Infect Dis 2020;ciaa1343. PubMed
- 8. Trifi A, Abdellatif S, Oueslati M, Zribi M, Daly F, Nasri R, et al. Nosocomial infections: current situation in a resuscitation-unit. Tunis Med 2017;95:179-84.PubMed
- 9. Schwendimann R, Blatter C, Dhaini S, Simon M, Ausserhofer D. The occurrence, types, consequences and preventability of in-hospital adverse events: a scoping review. BMC Health Serv Res 2018;18:521. ArticlePubMedPMCPDF
- 10. World Health Organization. Patient safety fact file [Internet]. World Health Organization. 2019;[cited 2023 Sep 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/patient-safety.
- 11. Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections: an overview. Infect Drug Resist 2018;11:2321-33.PubMedPMC
- 12. Peters A, Tartari E, Mai SH, Allegranzi B, Pittet D. 2019 WHO hand hygiene campaign and global survey: clean care for all-it's in your hands. Lancet Infect Dis 2019;19:463-4.ArticlePubMed
- 13. Saito H, Allegranzi B, Pittet D. 2018 WHO hand hygiene campaign: preventing sepsis in health care and the path to universal health coverage. Lancet Infect Dis 2018;18:490-2.ArticlePubMed
- 14. Roshan R, Feroz AS, Rafique Z, Virani N. Rigorous hand hygiene practices among health care workers reduce hospital-associated infections during the COVID-19 pandemic. J Prim Care Community Health 2020;11:2150132720943331. ArticlePubMedPMCPDF
- 15. Baccolini V, Migliara G, Isonne C, Dorelli B, Barone LC, Giannini D, et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: a retrospective cohort study. Antimicrob Resist Infect Control 2021;10:87. ArticlePubMedPMCPDF
- 16. Petty LA, Flanders SA, Vaughn VM, Ratz D, O'Malley M, Malani AN, et al. Risk factors and outcomes associated with community-onset and hospital-acquired coinfection in patients hospitalized for coronavirus disease 2019 (COVID-19): a multihospital cohort study. Infect Control Hosp Epidemiol 2022;43:1184-93.ArticlePubMed
- 17. Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2021;73:e445-54.ArticlePubMedPDF
- 18. He Y, Li W, Wang Z, Chen H, Tian L, Liu D. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol 2020;41:982-3.ArticlePubMed
- 19. Soriano MC, Vaquero C, Ortiz-Fernández A, Caballero A, Blandino-Ortiz A, de Pablo R. Low incidence of co-infection, but high incidence of ICU-acquired infections in critically ill patients with COVID-19. J Infect 2021;82:e20-1.ArticlePMC
- 20. Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest 2021;160:454-65.ArticlePubMed
- 21. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782-93.ArticlePubMed
- 22. Luyt CE, Bouadma L, Morris AC, Dhanani JA, Kollef M, Lipman J, et al. Pulmonary infections complicating ARDS. Intensive Care Med 2020;46:2168-83.ArticlePubMedPMCPDF
- 23. Kokkoris S, Papachatzakis I, Gavrielatou E, Ntaidou T, Ischaki E, Malachias S, et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect 2021;107:95-7.ArticlePubMed
- 24. Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J AntimicrobChemother 2021;76:1078-84.ArticlePDF
- 25. Bardi T, Pintado V, Gomez-Rojo M, Escudero-Sanchez R, Azzam Lopez A, Diez-Remesal Y, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis 2021;40:495-502.ArticlePubMedPMCPDF
- 26. Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 2021;27:83-8.ArticlePubMed
- 27. Cheng K, He M, Shu Q, Wu M, Chen C, Xue Y. Analysis of the risk factors for nosocomial bacterial infection in patients with COVID-19 in a tertiary hospital. Risk Manag Healthc Policy 2020;13:2593-9.PubMedPMC
- 28. Kyi M, Colman PG, Wraight PR, Reid J, Gorelik A, Galligan A, et al. Early intervention for diabetes in medical and surgical inpatients decreases hyperglycemia and hospital-acquired infections: a cluster randomized trial. Diabetes Care 2019;42:832-40.ArticlePubMedPDF
- 29. Khaodhiar L, McCowen K, Bistrian B. Perioperative hyperglycemia, infection or risk? Curr Opin Clin Nutr Metab Care 1999;2:79-82.ArticlePubMed
- 30. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-8.ArticlePubMedPMCPDF
Citations
Citations to this article as recorded by

 , Selim Sellaouti, Asma Mehdi, Lynda Messaoud, Eya Seghir, Badis Tlili, Sami Abdellatif
, Selim Sellaouti, Asma Mehdi, Lynda Messaoud, Eya Seghir, Badis Tlili, Sami Abdellatif 





 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite