Abstract
- During the coronavirus disease 2019 (COVID-19) pandemic, clinical staff learned how to manage patients enduring extended stays in an intensive care unit (ICU). COVID-19 patients requiring critical care in an ICU face a high risk of experiencing prolonged intensive care (PIC). The use of invasive mechanical ventilation in individuals with severe acute respiratory distress syndrome can cause numerous complications that influence both short-term and long-term morbidity and mortality. Those risks underscore the importance of proactively addressing functional complications. Mitigating secondary complications unrelated to the primary pathology of admission is imperative in minimizing the risk of PIC. Therefore, incorporating strategies to do that into daily ICU practice for both COVID-19 patients and those critically ill from other conditions is significantly important.
-
Keywords: COVID-19; critical care; physical therapy modalities; rehabilitation; SARS-COV2
INTRODUCTION
Prolonged intensive care (PIC) can be defined as a length of stay (LOS) of more than 10 days in an intensive care unit (ICU) irrespective of the cause of admission, and it is decisive in the outcomes of critically ill patients [1]. An LOS of >10 days increases in-hospital mortality and reduces the probability of returning home. Damuth et al. [1] reported a mortality rate of 62% 1 year after discharge from the ICU in patients who underwent mechanical ventilation for more than 14 days, and that rate reached up to 73% in ICUs across the United States [2]. Interestingly, patients with an initially high predicted risk of in-hospital death see a decline in their mortality rate after a 20-day hospital stay (77%–44.5%). Conversely, for patients initially deemed to have a low predicted risk of in-hospital death, their mortality rate increases after the same 20-day period (5.2 %-21.6%) [3]. Critically ill patients with prolonged hospital stays represent a low percentage of total ICU admissions (5%), generating 32.8% of total ICU days and 14.7% of hospital bed-days [1]. Kamdar et al. [2] reported that only 68% of ICU survivors had returned to work 42–60 months post-discharge, and 20%–36% lost their employment later. Therefore, prolonged ICU stays are a clinical and financial challenge for healthcare systems worldwide [4].
PIC can also be defined as the development of particular complications during an ICU stay. Chronic critical illness is often caused by persistent inflammation, immunosuppression, and catabolic syndrome, sepsis, or prolonged mechanical ventilation. Two types of patients can experience PIC: those whose admission pathology has a slow recovery process (e.g., coronavirus disease 2019 [COVID-19], severe acute respiratory distress syndrome (ARDS), acute brain injury, Guillain-Barre syndrome, and frailty syndrome) and those who present complications secondary to their ICU stay [5-7]. Failure in patient care begins when patients with a low probability of survival or those who require palliative care are selected for admission to the ICU because that selection betrays an unrealistic expectation about the probable evolution of the patient’s condition or disease. Errors in the general care of critically ill patients can predispose them to complications and increase the length of hospital stay and in-hospital mortality. Examples include excessive sedation, absence of structured early mobilization programs, poor ventilatory weaning protocols, delayed decision to perform tracheostomy, poor nutritional management, and healthcare-associated infections.
In critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, Roedl et al. [6] reported an LOS of ≥21 days in 55% of cases, and they classified those patients as receiving PIC. Those patients underwent an average of 30 days of mechanical ventilation. The overall ICU mortality of patients with severe SARS-CoV-2 infection was 50%, in contrast to 28% of non-COVID patients with an LOS longer than 21 days. Another study reported an LOS of 21 days in 75% to 80% of patients admitted to the ICU with severe SARS-CoV-2 infection. ARDS due to viral infection secondary to severe SARS-CoV-2 seemed to be a major risk factor for the development of PIC [8]. Systemic inflammatory processes, hypercoagulation, lung parenchymal damage, prolonged mechanical ventilation, immobility, and initial ignorance of the disease are the causes of those outcomes [6]. Associated infections are a major problem in this population [5]. The complexity of managing patients with COVID-19 severe enough to require ICU admission is a challenge for all health systems worldwide.
Risk factors associated with dependency in patients with COVID-19 include age, use of corticosteroids, time without out-of-bed mobilization, and hyperglycemia [6,9,10]. In critical COVID-19 patients who required mechanical ventilation, the only risk factors were lack of out-of-bed mobilization and time of profound sedation. Notably, in patients who need oxygen therapy and mechanical ventilation for this disease, corticosteroids play a massive role in treatment, but they carry their own risk for functional deterioration [11]. Furthermore, ARDS management includes profound sedation, neuromuscular blockers, immobility, and corticosteroids, which seems to be the perfect combination for establishing severe physical impairments [12,13]. Therefore, functional progression and early rehabilitation inside the ICU are needed to overcome the many risk factors and complications that critical COVID-19 patients can manifest. Functional recovery at discharge is closely related to patient strength at ICU discharge and ICU LOS [14].
Our primary objective for this review is to underscore the significance of rehabilitation, nutrition, and preventive measures in addressing complications within the ICU, including PIC. Also, we outline key elements of PIC management in COVID and non-COVID patients.
RISK FACTORS ASSOCIATED WITH PIC
The most important factors in PIC beyond critical pathology are previous chronic diseases, levels of functionality, muscle mass, and nutritional status [15]. Presently, the available evidence contains discrepancies concerning whether age and sarcopenia are risk factors for extended ICU stays. Notably, those two factors have predominantly been linked to in-hospital mortality [16,17]. However, an epidemiological study in the United States determined that the peak of chronic critical patients occurs between 75 and 79 years of age, with an incidence of 82.1 per 100,000 [18]. Sarcopenia increases the risk of difficult weaning (odds ratio [OR], 4.76) and in-hospital mortality (OR, 5.07) in critically ill patients [19]. Frailty syndrome also increases in-hospital mortality and decreases long-term lifespans, according to a meta-analysis published by Muscedere et al. [20] in 2017. Likewise, malnutrition and micronutrient depletion increase the risk of a prolonged hospital stay and the complications it generates from 20% to 40% [21].
Chronic heart, renal, and hepatic failure, along with lung diseases that make patients susceptible to extended mechanical ventilation, are recognized as risk factors for admission to the ICU, among other risk factors (Table 1). In contrast, obesity has been associated with lower 4-year mortality compared with patients with a normal or low weight, even though, paradoxically, patients with morbid obesity have longer ICU and hospital stays [22]. A critically ill patient's previous functional status should be included in the evaluation to determine the likelihood of in-hospital mortality and risk of complications, as when oncological patients are assessed using the Eastern Cooperative Oncology Group’s performance status [23].
Melamed et al. [9] reported interesting risk factors and associations in patients on prolonged ventilation during the COVID-19 pandemic. Among patients who underwent mechanical ventilation for >17 days, being male and direct admission to the ICU were risk factors even though that group had lower comorbidities according to the Charlson comorbidity index. In addition, patients who had prolonged mechanical ventilation in that study had a lower PaO2/FiO2 index and higher PaCO2, plateau pressure, and driving pressure during the first 2 weeks of mechanical ventilation, which are determinants of ARDS severity. That group also required a longer duration of neuromuscular blockade, prone position, and tracheostomy. Therefore, patients with longer mechanical ventilation had longer hospital stays. Surviving patients had a lower plateau pressure and higher PaO2/FiO2 at 2 weeks, with improvement in those parameters from weeks 1 to 2 [9].
MUSCULAR, FUNCTIONAL, AND NUTRITIONAL EVALUATION
When the human body is subjected to excessive and prolonged stress, such as in critical diseases, an imbalanced state called allostatic overload develops. It results in nutritional deprivation, excessive energy demand due to a persistent inflammatory state, and multiple organ failure. Such an overload is a determining factor in the incidence of chronic critical disease, and it manifests as muscle, functional, and nutritional deterioration [16,24]. Therefore, identification of allostatic overload, as well as evaluation of hemodynamic and ventilatory status, is vital when attending patients with PIC [16].
Muscle mass on admission is a predictor of prolonged ICU and hospital stays, according to a study conducted in Mexico. Surviving patients with low muscle mass on admission had 25 days of ventilation compared with 15 days for individuals with normal muscle mass; however, that difference was not statistically significant. In contrast, an ICU stay of 25 days and a hospital stay of 35 days, compared with 18 and 23 days, respectively, was a statistically significant difference between those groups. An association between low muscle mass on admission and tracheostomy requirements was also reported [25].
MUSCLE EVALUATION
Muscle wasting is a clinical manifestation of persistent inflammation, immunosuppression, and catabolic syndrome [26]. Severe muscle wasting is characterized by a loss of muscle mass and weakness, leading to the muscular dysfunction known as ICU-acquired weakness (ICUAW). This alteration is found in 32%–82% of patients exposed to prolonged mechanical ventilation, and it is especially common in elderly adults. Muscle wasting includes the respiratory muscles such as the diaphragm, and 80% of patients with severe wasting develop diaphragmatic dysfunction [27]. ICUAW and diaphragmatic dysfunction increase LOS, mechanical ventilation, weaning failure, care costs, morbidity, and in-hospital mortality [16]. Risk factors associated with ICUAW are predominant in every critical COVID-19 patient: immobility and the use of neuromuscular blockers and corticosteroids [28].
Among COVID-19 patients, a greater reduction in diaphragm and rectus femoris thickness was observed on ultrasonography from non-surviving patients. The echogenicity of both muscles was significantly increased at 7 days in surviving patients but was greater in non-surviving patients [29]. According to Nakanishi et al. [30], loss of biceps brachii muscle mass at 5 and 7 days is a predictor of in-hospital mortality in mechanically ventilated patients.
Different methods are used for muscle evaluation in critically ill patients, including those with critical COVID-19. Complex tools such as tomography, electrical impedance, and electrophysiological studies are the gold standards for diagnosing ICUAW [31], but more accessible tools, such as hand dynamometry (hand grip strength) and the Medical Research Council Sum Score (MRC-SS), are generally used [27]. High scores on such tools correlate with functional recovery at hospital discharge [14]. Quadriceps ultrasound can be very useful for determining muscle wasting throughout a patient’s stay [32,33]. Inspiratory muscles, such as the diaphragm, can also be evaluated with ultrasound using the diaphragmatic excursion and diaphragmatic thickening fraction [34-36].
FUNCTIONAL ASSESSMENT
Functional assessments of critically ill patients must begin with information about their functional status before hospital admission. Knowledge of previous functional status will help clinicians identify risk factors for developing PIC, such as sarcopenia, frailty, and level of independence in daily life activities [37]. Frailty and functional deterioration in critically ill COVID-19 patients allow clinicians to estimate their risk of developing new infections, organ failure, and poor long-term outcomes [28].
The importance of determining the risk of frailty has increased during the past 5 years because of its effects on the outcomes of critically ill patients and their families. Tools such as the frailty scale, clinical frailty scale, hospital frailty scale (>5 points), and Katz index (<6 points) should guide preventive healthcare decisions, such as the early start of rehabilitation programs, integration of geriatric services, and palliative and end-of-life care [38,39]. Currently validated scales for critically ill patients provide valuable information about not only muscle strength but also functional status, such as the ability to sit, stand, or walk. Currently, a variety of instruments can evaluate functionality, such as the ICU Mobility Scale (IMS), Functional Status Score for the Intensive Care Unit, Chelsea Critical Care Physical Assessment Tool, Physical Function in Intensive Care Test (PFIT), and Perme Score [40].
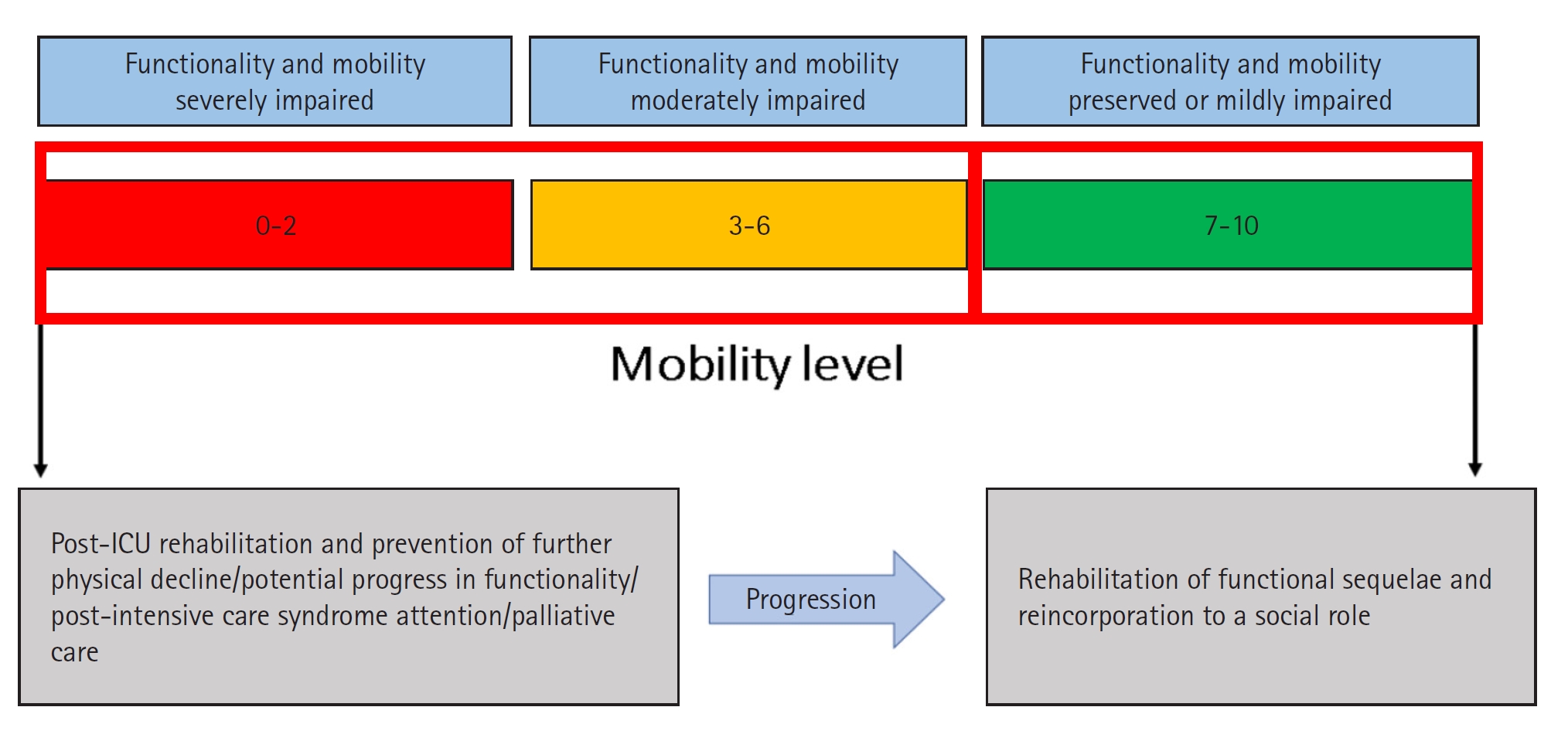
The IMS is composed of 11 items scored from a minimum of 0 to a maximum of 10 and is suitable for any stage of critical illness (Table 2). It is an outstanding tool because it has predictive validity for hospital discharge and survival at 90 days [41]. It has also been correlated with the MRC-SS, PFIT, and Perme Score [40]. Because of the speed and ease of its application, the IMS can be performed daily regardless of the severity of the patient and can guide physiotherapeutic interventions in the ICU to meet functional objectives. An additional benefit is that it gives nursing staff information about patient mobility and the degree of care required. The IMS is a good option for evaluating the mobility of critically ill patients in an environment with restricted resources, large numbers of patients, or few physical therapy personnel [42]. A color coding scheme is proposed to quickly classify patients with high, moderate, and low levels of mobility (Figure 1). Patients with a level >3 will be able to stand, which considerably facilitates nursing care at any clinical stage and reduces complications derived from elongated decubitus.
Muscular strength at discharge from the ICU is important; however, recognizing the level of mobility and functionality allows clinicians to establish interventions and treatment to facilitate a higher level of independence. Some post-ICU evaluations, such as the 6-minute walk test, are not feasible for patients with low mobility. Functionality categorization should be integrated into the daily assessment of critically ill patients to achieve a complete and well-rounded evaluation.
According to an international survey, more than 90% of patients with and without COVID-19 who required invasive mechanical ventilation remained in bed without mobilization, even though a COVID-19 diagnosis does not seem to be a barrier to early mobilization [43]. This finding highlights the need to improve the processes of functional assessment, early mobilization, and prevention of functional sequelae in patients under mechanical ventilation, regardless of their diagnosis [8,43].
NUTRITIONAL ASSESSMENT
Evaluating the nutritional status of patients in the ICU allows the identification of alterations such as malnutrition [44]. Patients admitted to the ICU for longer than 48 hours are at risk of malnutrition [45]. Tools for the timely identification of the presence or risk of nutritional alterations, such as the Nutrition Risk Score and Nutrition Risk in the Critically Ill, have been validated [46,47]. A thorough evaluation involving clinical history, clinical signs, physical examination, and anthropometry is recommended to identify malnutrition, mainly by assessing the loss of muscle mass, laboratory values, dietary information, and functionality.
Another tool for the diagnosis of malnutrition is GLIM (Global Leadership Initiative on Malnutrition), developed in a global consensus by American Society for Parenteral and Enteral (ASPEN), European Society for Clinical Nutrition and Metabolism (ESPEN), Latin-American Federation of Nutritional Therapy, Clinical Nutritionand Metabolism (FELANPE) and Parenteral and Enteral Nutrition Society of Asia (PENSA) to prioritize the early diagnosis of malnutrition. GLIM considers three phenotypic criteria (weight loss (%), body mass index, and reduction of muscle mass) and two etiological criteria (reduction of intake or assimilation and inflammation) [48]. A diagnosis of malnutrition requires at least one phenotypic criterion and one etiological criterion (Table 3). An update of this concept proposes that skeletal muscle function be measured using techniques such as electrical bioimpedance analysis, dual-energy X-ray absorptiometry, computed tomography, ultrasound, calf circumference, physical examination, and mean arm circumference [48-50]. For this reason, ultrasound has gained relevance in clinical practice for identifying and monitoring muscle and nutritional status because of its low cost, safety, and straightforward application [33,36,51].
After a nutritional evaluation, nutritional diagnoses are made to determine the intervention. Enteral nutrition should be prioritized to maintain gastrointestinal and immunological homeostasis [52]. Complete nutrition in the acute phase does not provide a mortality advantage and can be harmful. The initiation of nutritional therapy must be individualized according to the patient’s nutritional status before admission, severity, and stage of disease [45].
In the ICU, nutrition should be initiated as soon as possible (within 48 hours) unless there are significant contraindications (hemodynamic instability is the most common) [49,52]. Enteral nutrition in critical patients requiring vasopressors and mechanical support can be feasible and safe, provided that the resuscitation stage is completed and adequate supervision is provided [53]. Currently, using technology and objective methods to accurately assess body composition and estimate the nutritional requirements of critically ill patients is a therapeutic alternative to early nutrition initiation. Critically ill patients can experience refeeding syndrome. To prevent it, the early administration of thiamin, trophic contributions during the first days of the ICU stay, and electrolyte monitoring can be helpful, but the most important factor is individualized nutrition [54].
During the pandemic, malnutrition due to decreased food intake and systemic inflammation led to severe muscle loss and liver dysfunction [50]. Muscular impairments associated with severe SARS-COV-2 infection affected the quality of life of patients and their families by causing disability, dependency, and increased morbidity upon discharge from the ICU [50,55]. In addition, malnutrition, cachexia, and loss of muscle mass can result from respiratory distress syndrome. Thus, anorexia, inflammation, hypermetabolism, muscle catabolism, and prolonged periods of immobility lead to both muscle atrophy and a longer duration of mechanical ventilation [56]. Providing suitable nutrition for patients with severe SARS-COV-2 infection is challenging because of their complex and delicate state. Some precise recommendations were made during this period and are listed in Table 4 [49,50,56].
MANAGING PIC
The catastrophic consequences of critical illness have been described for several years. In patients with ARDS due to COVID-19, the risk of PIC is high and can lead to the development of future disabilities and death even outside the hospital [28,57,58]. Preventive measures for these issues have been well-described in the literature [59-64]. However, the physio-pathological characteristics of critical COVID-19 and the risk factors associated with it and its management require strict and well-timed preventive measures, such as minimizing the time required for sedation and neuromuscular blocker administration, optimizing nutrition, and early mobilization [65].
In critical COVID-19 survivors, muscular strength and ICU LOS are determining factors in physical recovery [14]. Structured rehabilitation programs are needed to achieve full-fledged management of critically ill patients, including those with critical COVID-19. Therefore, the development of a multidisciplinary team that includes nutritionists and rehabilitation personnel will enable hospitals to provide functional-based treatment and not survival-centered management of critical illness. The ABCDEFG bundle is a group of strategies with sufficient evidence to be deemed necessary for the care and treatment of all critically ill patients [10]. However, when we talk about PIC, we encounter various challenges such as (1) a lack of scientific information, (2) insufficient knowledge of the subject by ICU staff, and (3) dismissal of the subject as unimportant by healthcare personnel. Several authors, such as Prescott and Madrid, have reported interventions focused on the care of critically ill patients and post-sepsis patients that can improve clinical thinking about the management of PIC [10,53].
Broadly speaking, patients undergoing PIC face a situation similar to that of critical patients, but they are much more vulnerable. Immunosuppression, muscle weakness, prolonged ventilation, and multiple surgical interventions are probable complications in these patients [53]. It is important to bear in mind that, although the patient is extremely vulnerable, all interventions must be chosen with extreme care to prevent infections, iatrogenic events, or any complications that could compromise the progression and health status of the patient.
A multidisciplinary team plays a fundamental role in such situations. The nurturing role, rehabilitation, nursing care, psychological support, and medical prescriptions must be synergistic in managing long-stay patients. All personnel treating a patient during a prolonged LOS should always have in mind that the main objective is to discharge the patient from the ICU, either to complete their hospital care and be re-integrated into society or to die in the most humane and dignified way possible [66-68].
To address PIC, ICU health providers must ask whether the reason a patient cannot leave the ICU is the same as their reason for admission. The answer will help them identify whether the PIC is caused by the natural history of the disease or complications from the ICU care itself. A patient’s functional status in the ICU can offer a different approach to decision-making during the progression of a critical patient because it allows all providers to see the patient in a comprehensive and empathetic way [40]. Timely identification of complications, such as ICU-acquired weakness, diaphragmatic dysfunction induced by mechanical ventilation, malnutrition, immune paralysis, and persistent inflammation, will allow ICU staff to recognize high-risk patients [5-7,53]. We have developed algorithms to simplify the prevention, management, and critical aspects of PIC in critically ill COVID and non-COVID patients (Figures 2 and 3) [61,63,65,69-74].
The main interventions from which these patients would benefit are described in Table 5 [65,74,75]. It is worth mentioning that these interventions are not new to any health provider in the ICU. However, efforts should be focused on preventing infections, improving function, feeding properly, and empowering the family to meet the basic premise, or the patient will never be able to leave the ICU (Figure 4). Functional impairment caused by COVID-19 is a challenge for health systems worldwide. Rehabilitation programs are a priority to minimize the effects of the pandemic and any upcoming pathologies that can lead to any type of disability [10].
CONCLUSIONS
The management of COVID-19 patients posed a formidable challenge to our multidisciplinary ICU team. Given the gravity of this critical illness and the prevailing pandemic conditions, it has become imperative to devise strategies to mitigate the functional decline often observed in ICU survivors. Consequently, across the globe, coordinated multidisciplinary teams have emerged in response to this global crisis, with the primary goal of preventing and restoring functional impairments. Today, the focus in caring for critically ill patients extends beyond mere survival; it encompasses the restoration of individuals to their pre-illness quality of life, regardless of whether the patient is diagnosed with COVID or any other critical condition.
KEY MESSAGES
▪ Today, the focus in caring for critically ill patients extends beyond mere survival.
▪ It encompasses the restoration of individuals to their pre-illness quality of life, regardless of whether the patient is diagnosed with coronavirus disease or any other critical condition.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: MAMC. Visualization: RAJB. Project administration: AAPC. Writing–original draft: all authors. Writing–review & editing: all authors.
-
ACKNOWLEDGMENTS
We extend our sincere thanks to the authorities of Hospital General de Mexico "Dr. Eduardo Liceaga" (General Hospital of Mexico) for their invaluable support, resources, and cooperation, which greatly facilitated this research.
Figure 1.Intensive care unit Mobility Scale (IMS) color coding. ICU: intensive care unit.
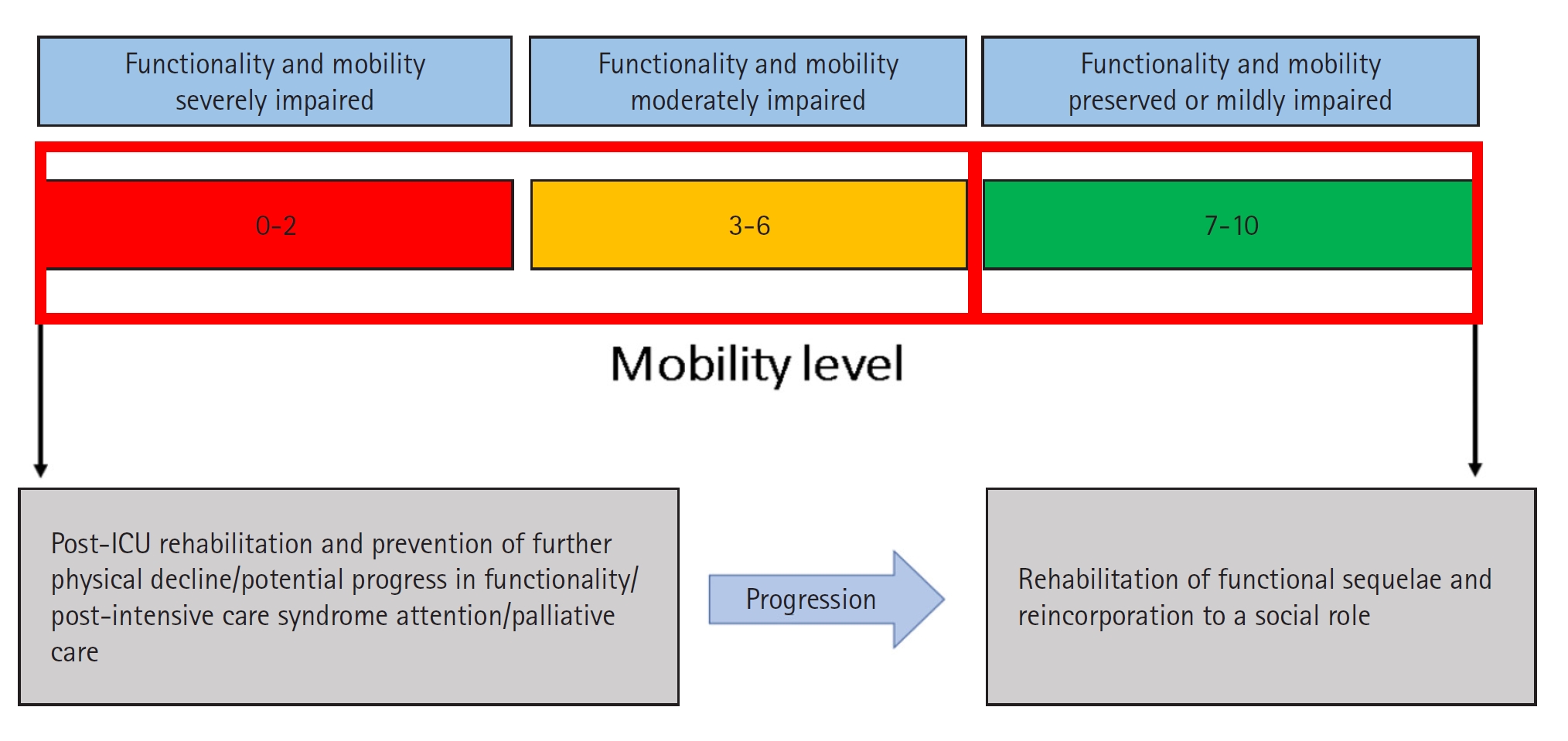
Figure 2.Prevention and management of prolonged intensive care (PIC) for critically ill patients. ICU: intensive care unit; SAT: spontaneous awakening trial; SBT: spontaneous breathing trial; RASS: Richmond Agitation-Sedation Scale; NMES: neuromuscular electrical stimulation; FES: functional electrical stimulation.
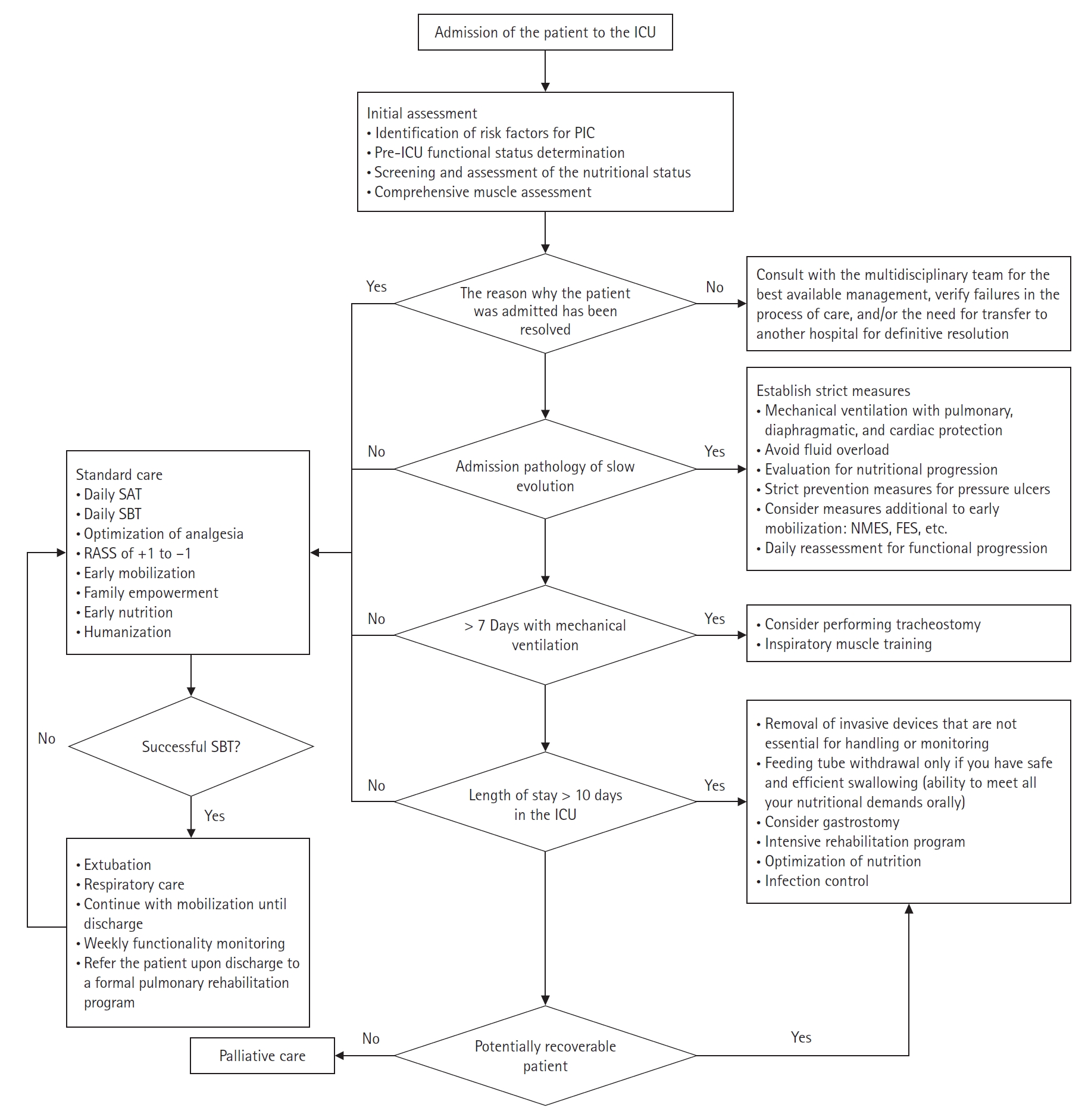
Figure 3.Prevention and management of prolonged intensive care (PIC) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. ICU: intensive care unit; COVID-19: coronavirus disease 2019; SAT: spontaneous awakening trial; SBT: spontaneous breathing trial; RASS: Richmond Agitation-Sedation Scale.
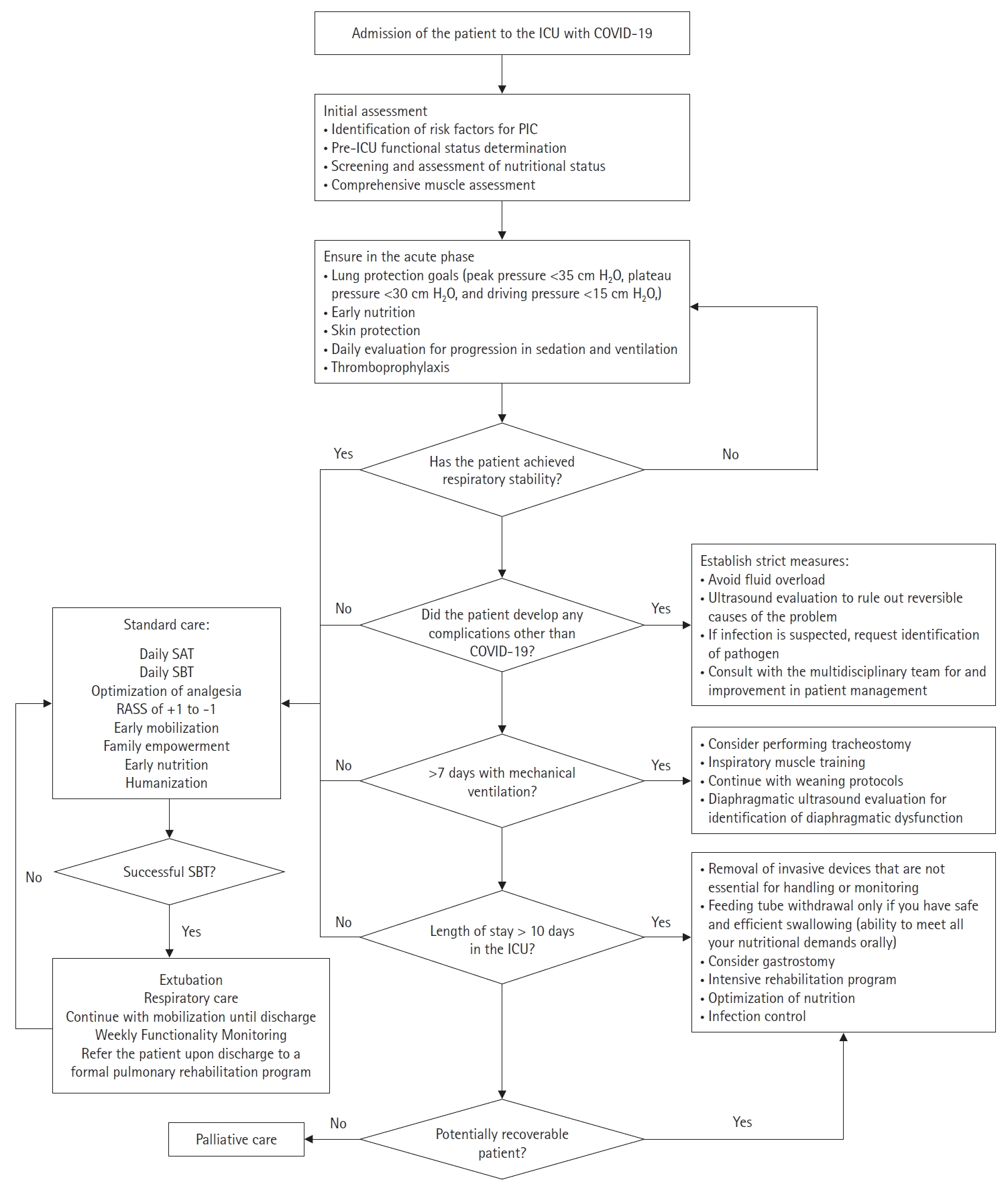
Figure 4.Risk factors and interventions for prolonged intensive care. COPD: chronic obstructive pulmonary disease.

Table 1.Risk factors for prolonged intensive care at hospital or intensive care unit admission
|
Risk factor |
|
∙ Glasgow coma scale score ≤8 |
|
∙ APACHE II score ≥9 |
|
∙ SOFA score >3 |
|
∙ Immunocompromise |
|
∙ Elderly age |
|
∙ Nutritional alterations (BMI ≤18.5 or ≥30 kg/m2) |
|
∙ Neurological and neuromuscular disease |
|
∙ Chronic degenerative disease (diabetes, COPD, kidney, heart disease, among others) |
|
∙ Exacerbation of a progressive underlying disease |
|
∙ Need for prolonged mechanical ventilation |
|
∙ Anemia |
|
∙ Impaired functional status on admission |
|
∙ Severe ARDS |
|
Table 2.Intensive care unit Mobility Scale
|
Level |
Classification |
Definition |
|
0 |
Nothing (lying in bed) |
Passively rolled or passively exercised by staff but not actively moving. |
|
1 |
Sitting in bed, exercises in bed |
Any activity in bed, including rolling, bridging, active exercises, cycle ergometry, and active assisted exercises; not moving out of bed or over the edge of the bed. |
|
2 |
Passively moved to chair (no standing) |
Hoist, passive lift, or slide transfer to a chair, with no standing or sitting on the edge of the bed. |
|
3 |
Sitting over edge of bed |
May be assisted by staff, but involves actively sitting over the side of the bed with some trunk control. |
|
4 |
Standing |
Weight bearing through the feet in the standing position, with or without assistance. This may include use of a standing lifter device or tilt table. |
|
5 |
Transferring bed to chair |
Able to step or shuffle through standing to the chair. This involves actively transferring weight from one leg to another to move to the chair. If the patient has been stood with the assistance of a medical device, they must step to the chair (not included if the patient is wheeled in a standing lifter device). |
|
6 |
Marching on spot (at bedside) |
Able to walk on the spot by lifting alternate feet (must be able to step at least 4 times, twice on each foot), with or without assistance. |
|
7 |
Walking with assistance of 2 or more people |
Walking away from the bed/chair by at least 5 m (5 yards) assisted by 2 or more people. |
|
8 |
Walking with assistance of 1 person |
Walking away from the bed/chair by at least 5 m (5 yards) assisted by 1 person. |
|
9 |
Walking independently with a gait aid |
Walking away from the bed/chair by at least 5 m (5 yards) with a gait aid, but no assistance from another person. In a wheelchair-bound person, this activity level includes wheeling the chair independently 5 m (5 years) away from the bed/chair. |
|
10 |
Walking independently without a gait aid |
Walking away from the bed/chair by at least 5 m (5 yards) without a gait aid or as similation from another person. |
Table 3.Phenotypic and etiological criteria for the diagnosis of malnutrition criteria in critically ill patients
|
Phenotypic criteria |
Etiological criteria |
|
Unintentional weight loss |
Low body mass index (kg/m2) |
Reduced muscle mass |
Decreased food intake or assimilation |
Inflammatory load |
|
>5% in the past 6 months or >10% in more than 6 months |
<20 in people <70 years or <22 in people >70 years |
As shown by validated body composition techniques |
<50% >1 week or <100% >2 weeks or chronic gastrointestinal condition that alters assimilation |
Acute injury/inflammation, chronic inflammatory pathology |
Table 4.International recommendations for nutrition in critically ill patients during the COVID-19 era
|
Guide |
Total intake |
Protein intake |
|
ESPEN (2019) |
Use indirect calorimetry |
1.3 g/kg/day |
|
Simple formula: 20–25 kcal/kg/day |
|
|
Alternative: CO2 production in ventilator (REE=VCO2×8.19) |
Early acute phase: 0.8 g/ideal weight/day |
|
Early acute phase: 20 kcal/ideal weight |
Late acute phase: 0.8–1.3 g/ideal weight |
|
Late acute phase: 25 kcal/ideal weight |
Chronic phase: >2.5 g/ideal weight |
|
Chronic phase: 25 kcal/ideal weight |
|
|
ASPEN (2022) |
Indirect calorimetry |
1.2–2 g/kg/day |
|
12–25 kcal/kg/day (in the first 7–10 days in the ICU) |
|
SEMICYUC (2020) |
Indirect calorimetry |
1.2–2 g/kg/day |
|
25–30 kcal/kg/day |
Obese patient |
|
Obese patient, adjusted weight |
BMI 30–40 kg/m2: 1.8–2 g/kg (ideal weight)/day |
|
BMI >40 kg/m2: 2.1–2.5 g/kg (ideal weight)/day |
|
ASPEN/ESPEN obese patient |
BMI >30–50 kg/m2: 11–14 kcal/actual weight/day |
2.0–2.5 g/ideal weight/day |
|
BMI 30–50 kg/m2: 18–22 kcal/ideal weight |
1.3 g/ideal weight |
|
BMI >50 kg/m2: 22–25 kcal/weight |
|
|
Acute kidney injury or chronic kidney disease |
Protein |
|
Critically ill patients without renal function replacement therapy: 1.3 g/weight |
|
Critically ill patients with intermittent renal function replacement therapy: 1.3–1.5 g/weight |
|
Patients with substitutive treatment of renal function |
|
ASPEN: 1.7–2.5 g/weight |
|
ESPEN: 1.5–1.7 g/weight |
|
COVID-19 |
ASPEN |
ASPEN |
|
15–20 kcal/current weight/day in the first week, increase requirements to 50%–70% on the second day, reach 80%–100% on the fourth day |
1.2–2.0 g/weight/day |
|
ESPEN |
ESPEN |
|
27 kcal/weight/day >65 years with comorbidities |
1.0 g/weight/day |
|
30 kcal/weight/day with comorbidities and low weight (gradual increase) |
>1.0 g/weight/day in hospitalized patients to prevent loss of body weight and reduce complications and ICU readmissions |
|
30 kcal/weight/day in older adults, adjusted for nutritional status, physical activity, disease stage, and nutritional tolerance |
Severe patients 1.3 g/kg/day (achieved between the third and fifth day) |
Table 5.Interventions to prevent and manage prolonged intensive care
|
Intervention |
Benefit |
|
Family empowerment |
∙ Humanize care |
|
∙ Establish continuous communication with the family and the patient |
|
∙ Mitigate psychological disorders such as PTSD |
|
∙ Create a real vision of the patient’s health status |
|
∙ Reduce delirium |
|
Prioritize early physical therapy |
∙ Reduce ICU-acquired weakness |
|
∙ Improve respiratory capacity |
|
∙ Prevent dependence on mechanical ventilation |
|
∙ Promote functionality |
|
∙ Reduce frailty |
|
∙ Provide motivational factor |
|
Aggressive sepsis management |
∙ Prevent reinfection and shock |
|
∙ Reduce readmissions |
|
∙ Improve prognosis |
|
Constant ventilatory challenges |
∙ Push the patient to maximum respiratory capacity |
|
∙ Reduce the time connected to mechanical ventilation and prevent ventilator-associated pneumonia |
|
∙ Avoid perpetuating mechanical ventilation |
|
∙ Reduce ventilatory dependence |
|
Optimal nutrition and immuno-nutrition |
∙ Improve muscle quality and reinforce physical therapy |
|
∙ Reduce the presence of cachexia |
|
∙ Improve energy reserves |
|
∙ Reduce immunodeficiency |
|
Psychological approach |
∙ Reduce incidence of mental disorders |
|
∙ Humanize the service |
|
∙ Improve communication with the patient |
|
∙ Prepare everyone for unfavorable outcomes |
|
Early tracheostomy (≤7 days of mechanical ventilation) |
∙ Comfort for the patient |
|
∙ Prevent atrophy of facial, lingual, velar, pharyngeal, and laryngeal muscles |
|
∙ Prevent loss of function of the upper airway (phonation, cough, swallowing, and management of secretions) |
|
∙ Reduce days of mechanical ventilation |
|
∙ Reduce days of hospital stay |
|
∙ Reduce costs of care related to the reduction of hospital stay |
References
- 1. Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med 2015;3:544-53.ArticlePubMed
- 2. Kamdar BB, Suri R, Suchyta MR, Digrande KF, Sherwood KD, Colantuoni E, et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax 2020;75:17-27.ArticlePubMed
- 3. Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med 2016;4:566-73.ArticlePubMed
- 4. Iwashyna TJ, Viglianti EM. Patient and population-level approaches to persistent critical illness and prolonged intensive care unit stays. Crit Care Clin 2018;34:493-500.ArticlePubMedPMC
- 5. Chatterjee SK, Saha S, Munoz MN. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front Mol Biosci 2020;7:196. ArticlePubMedPMC
- 6. Roedl K, Jarczak D, Boenisch O, de Heer G, Burdelski C, Frings D, et al. Chronic critical illness in patients with COVID-19: characteristics and outcome of prolonged intensive care therapy. J Clin Med 2022;11:1049. ArticlePubMedPMC
- 7. Voiriot G, Oualha M, Pierre A, Salmon-Gandonnière C, Gaudet A, Jouan Y, et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann Intensive Care 2022;12:58. ArticlePubMedPMCPDF
- 8. Bonorino KC, Cani KC. Early mobilization in the time of COVID-19. Rev Bras Ter Intensiva 2020;32:484-6.ArticlePubMedPMC
- 9. Melamed R, Paz F, Jepsen S, Smith C, Saavedra R, Mulder M, et al. Prognostic factors and outcomes in COVID-19 patients requiring prolonged mechanical ventilation: a retrospective cohort study. Ther Adv Respir Dis 2022;16:17534666221086415. ArticlePubMedPMCPDF
- 10. Nakanishi N, Liu K, Kawakami D, Kawai Y, Morisawa T, Nishida T, et al. Post-intensive care syndrome and its new challenges in coronavirus disease 2019 (COVID-19) pandemic: a review of recent advances and perspectives. J Clin Med 2021;10:3870. ArticlePubMedPMC
- 11. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JA, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330-41.PubMed
- 12. Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019;9:69. ArticlePubMedPMCPDF
- 13. Yang T, Li Z, Jiang L, Wang Y, Xi X. Risk factors for intensive care unit-acquired weakness: a systematic review and meta-analysis. Acta Neurol Scand 2018;138:104-14.ArticlePubMedPDF
- 14. Stripari Schujmann D, Claudia Lunardi A, Neri Peso C, Pompeu JE, Annoni R, Miura MC, et al. Functional recovery groups in critically ill COVID-19 patients and their associated factors: from ICU to hospital discharge. Crit Care Med 2022;50:1799-808.ArticlePubMedPMC
- 15. Soto GL. The chronic critical patient. Rev Med Clin Condes 2019;30:160-70.
- 16. Loss SH, Nunes DS, Franzosi OS, Salazar GS, Teixeira C, Vieira SR. Chronic critical illness: are we saving patients or creating victims? Rev Bras Ter Intensiva 2017;29:87-95.ArticlePubMedPMC
- 17. Baggerman MR, van Dijk DP, Winkens B, van Gassel RJ, Bol ME, Schnabel RM, et al. Muscle wasting associated co-morbidities, rather than sarcopenia are risk factors for hospital mortality in critical illness. J Crit Care 2020;56:31-6.ArticlePubMed
- 18. Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, et al. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015;43:282-7.ArticlePubMedPMC
- 19. Kou HW, Yeh CH, Tsai HI, Hsu CC, Hsieh YC, Chen WT, et al. Sarcopenia is an effective predictor of difficult-to-wean and mortality among critically ill surgical patients. PLoS One 2019;14:e0220699.ArticlePubMedPMC
- 20. Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med 2017;43:1105-22.ArticlePubMedPMCPDF
- 21. Instituto Nacional de Estadística y Geografía. Encuesta Nacional de Salud y Nutrición 2018: diseño conceptual. Instituto Nacional de Salud Pública. 2018.
- 22. Zhou D, Wang C, Lin Q, Li T. The obesity paradox for survivors of critically ill patients. Crit Care 2022;26:198. ArticlePubMedPMCPDF
- 23. Azevedo LC, Caruso P, Silva UV, Torelly AP, Silva E, Rezende E, et al. Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest 2014;146:257-66.ArticlePubMed
- 24. Salahuddin N, Sammani M, Hamdan A, Joseph M, Al-Nemary Y, Alquaiz R, et al. Fluid overload is an independent risk factor for acute kidney injury in critically ill patients: results of a cohort study. BMC Nephrol 2017;18:45. ArticlePubMedPMCPDF
- 25. Osuna-Padilla IA, Rodríguez-Moguel NC, Rodríguez-Llamazares S, Orsso CE, Prado CM, Ríos-Ayala MA, et al. Low muscle mass in COVID-19 critically-ill patients: prognostic significance and surrogate markers for assessment. Clin Nutr 2022;41:2910-7.ArticlePubMedPMC
- 26. Meduri GU, Chrousos GP. General adaptation in critical illness: glucocorticoid receptor-alpha master regulator of homeostatic corrections. Front Endocrinol (Lausanne) 2020;11:161. ArticlePubMedPMC
- 27. Wang W, Xu C, Ma X, Zhang X, Xie P. Intensive care unit-acquired weakness: a review of recent progress with a look toward the future. Front Med (Lausanne) 2020;7:559789. ArticlePubMedPMC
- 28. Kho ME, Rewa OG, Boyd JG, Choong K, Stewart GC, Herridge MS. Outcomes of critically ill COVID-19 survivors and caregivers: a case study-centred narrative review. Can J Anaesth 2022;69:630-43.ArticlePubMedPMCPDF
- 29. Umbrello M, Guglielmetti L, Formenti P, Antonucci E, Cereghini S, Filardo C, et al. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19-related ARDS. Nutrition 2021;91-92:111449. ArticlePubMed
- 30. Nakanishi N, Oto J, Tsutsumi R, Akimoto Y, Nakano Y, Nishimura M. Upper limb muscle atrophy associated with in-hospital mortality and physical function impairments in mechanically ventilated critically ill adults: a two-center prospective observational study. J Intensive Care 2020;8:87. ArticlePubMedPMCPDF
- 31. Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med 2020;46:637-53.ArticlePubMedPMCPDF
- 32. Formenti P, Umbrello M, Coppola S, Froio S, Chiumello D. Clinical review: peripheral muscular ultrasound in the ICU. Ann Intensive Care 2019;9:57. ArticlePubMedPMCPDF
- 33. Toledo DO, Freitas BJ, Dib R, Pfeilsticker FJ, Santos DM, Gomes BC, et al. Peripheral muscular ultrasound as outcome assessment tool in critically ill patients on mechanical ventilation: an observational cohort study. Clin Nutr ESPEN 2021;43:408-14.ArticlePubMed
- 34. Haaksma ME, Smit JM, Boussuges A, Demoule A, Dres M, Ferrari G, et al. EXpert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care 2022;26:99. ArticlePubMedPMCPDF
- 35. Schreiber A, Bertoni M, Goligher EC. Avoiding respiratory and peripheral muscle injury during mechanical ventilation: diaphragm-protective ventilation and early mobilization. Crit Care Clin 2018;34:357-81.PubMed
- 36. Looijaard WG, Molinger J, Weijs PJ. Measuring and monitoring lean body mass in critical illness. Curr Opin Crit Care 2018;24:241-7.ArticlePubMedPMC
- 37. Jung C, Guidet B, Flaatten H, VIP study group. Frailty in intensive care medicine must be measured, interpreted and taken into account! Intensive Care Med 2023;49:87-90.ArticlePubMedPDF
- 38. Bruno RR, Wernly B, Flaatten H, Fjølner J, Artigas A, Baldia PH, et al. The association of the activities of daily living and the outcome of old intensive care patients suffering from COVID-19. Ann Intensive Care 2022;12:26. PubMedPMC
- 39. Subramaniam A, Ueno R, Tiruvoipati R, Srikanth V, Bailey M, Pilcher D. Comparison of the predictive ability of clinical frailty scale and hospital frailty risk score to determine long-term survival in critically ill patients: a multicentre retrospective cohort study. Crit Care 2022;26:121. ArticlePubMedPMCPDF
- 40. Parry SM, Huang M, Needham DM. Evaluating physical functioning in critical care: considerations for clinical practice and research. Crit Care 2017;21:249. ArticlePubMedPMCPDF
- 41. Tipping CJ, Bailey MJ, Bellomo R, Berney S, Buhr H, Denehy L, et al. The ICU mobility scale has construct and predictive validity and is responsive. a multicenter observational study. Ann Am Thorac Soc 2016;13:887-93.ArticlePubMed
- 42. Tipping CJ, Holland AE, Harrold M, Crawford T, Halliburton N, Hodgson CL. The minimal important difference of the ICU mobility scale. Heart Lung 2018;47:497-501.ArticlePubMed
- 43. Liu K, Nakamura K, Kudchadkar SR, Katsukawa H, Nydahl P, Ely EW, et al. Mobilization and rehabilitation practice in ICUs during the COVID-19 pandemic. J Intensive Care Med 2022;37:1256-64.ArticlePubMedPMCPDF
- 44. Arabi YM, Casaer MP, Chapman M, Heyland DK, Ichai C, Marik PE, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med 2017;43:1239-56.ArticlePubMedPMCPDF
- 45. Hill A, Elke G, Weimann A. Nutrition in the intensive care unit-A narrative review. Nutrients 2021;13:2851. PubMedPMC
- 46. Bolayir B, Arik G, Yeşil Y, Kuyumcu ME, Varan HD, Kara Ö, et al. Validation of Nutritional Risk Screening-2002 in a hospitalized adult population. Nutr Clin Pract 2019;34:297-303.ArticlePubMedPDF
- 47. de Vries MC, Koekkoek WK, Opdam MH, van Blokland D, van Zanten AR. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr 2018;72:428-35.ArticlePubMedPDF
- 48. Cederholm T, Jensen GL, Correia MI, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1-9.PubMed
- 49. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79.ArticlePubMed
- 50. Thibault R, Seguin P, Tamion F, Pichard C, Singer P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Crit Care 2020;24:447. ArticlePubMedPMCPDF
- 51. Nakanishi N, Tsutsumi R, Okayama Y, Takashima T, Ueno Y, Itagaki T, et al. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care 2019;7:61. ArticlePubMedPMCPDF
- 52. Preiser JC, van Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care 2015;19:35. ArticlePubMedPMCPDF
- 53. Hawkins RB, Raymond SL, Stortz JA, Horiguchi H, Brakenridge SC, Gardner A, et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front Immunol 2018;9:1511. ArticlePubMedPMC
- 54. da Silva JS, Seres DS, Sabino K, Adams SC, Berdahl GJ, Citty SW, et al. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract 2020;35:178-95.ArticlePubMedPDF
- 55. Cereda E, Clavé P, Collins PF, Holdoway A, Wischmeyer PE. Recovery focused nutritional therapy across the continuum of care: learning from COVID-19. Nutrients 2021;13:3293. ArticlePubMedPMC
- 56. Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr 2020;39:1631-8.ArticlePubMedPMC
- 57. Cavalleri J, Treguier D, Deliège T, Gurdebeke C, Ernst M, Lambermont B, et al. One-year functional decline in COVID-19 and non-COVID-19 critically ill survivors: a prospective study incorporating a pre-ICU status assessment. Healthcare (Basel) 2022;10:2023. ArticlePubMedPMC
- 58. Hodgson CL, Higgins AM, Bailey MJ, Mather AM, Beach L, Bellomo R, et al. The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care 2021;25:382. ArticlePubMedPMCPDF
- 59. Cameron S, Ball I, Cepinskas G, Choong K, Doherty TJ, Ellis CG, et al. Early mobilization in the critical care unit: a review of adult and pediatric literature. J Crit Care 2015;30:664-72.ArticlePubMed
- 60. Lipshutz AK, Engel H, Thornton K, Gropper MA. Early mobilization in the intensive care unit: evidence and implementation. ICU Dir 2012;3:10-6.
- 61. Zang K, Chen B, Wang M, Chen D, Hui L, Guo S, et al. The effect of early mobilization in critically ill patients: a meta-analysis. Nurs Crit Care 2020;25:360-7.ArticlePubMedPDF
- 62. Olkowski BF, Shah SO. Early mobilization in the neuro-ICU: how far can we go? Neurocrit Care 2017;27:141-50.ArticlePubMedPDF
- 63. Clarissa C, Salisbury L, Rodgers S, Kean S. Early mobilisation in mechanically ventilated patients: a systematic integrative review of definitions and activities. J Intensive Care 2019;7:3. ArticlePubMedPMCPDF
- 64. Paton M, Chan S, Tipping CJ, Stratton A, Serpa Neto A, Lane R, et al. The effect of mobilization at 6 months after critical illness: meta-analysis. NEJM Evid 2023;2:EVIDoa2200234. ArticlePubMed
- 65. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825-73.PubMed
- 66. Pan H, Shi W, Zhou Q, Chen G, Pan P. Palliative care in the intensive care unit: not just end-of-life care. Intensive Care Res 2023;3:77-82.ArticlePDF
- 67. Ito K, George N, Wilson J, Bowman J, Aaronson E, Ouchi K. Primary palliative care recommendations for critical care clinicians. J Intensive Care 2022;10:20. ArticlePubMedPMCPDF
- 68. Vincent JL. The continuum of critical care. Crit Care 2019;23(Suppl 1):122. ArticlePubMedPMCPDF
- 69. Zhang L, Hu W, Cai Z, Liu J, Wu J, Deng Y, et al. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS One 2019;14:e0223185.ArticlePubMedPMC
- 70. Ding N, Zhang Z, Zhang C, Yao L, Yang L, Jiang B, et al. What is the optimum time for initiation of early mobilization in mechanically ventilated patients?: a network meta-analysis. PLoS One 2019;14:e0223151.ArticlePubMedPMC
- 71. Menges D, Seiler B, Tomonaga Y, Schwenkglenks M, Puhan MA, Yebyo HG. Systematic early versus late mobilization or standard early mobilization in mechanically ventilated adult ICU patients: systematic review and meta-analysis. Crit Care 2021;25:16. ArticlePubMedPMCPDF
- 72. Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019;47:3-14.PubMedPMC
- 73. Arias-Fernández P, Romero-Martin M, Gómez-Salgado J, Fernández-García D. Rehabilitation and early mobilization in the critical patient: systematic review. J Phys Ther Sci 2018;30:1193-201.ArticlePubMedPMC
- 74. Tanaka A, Uchiyama A, Kitamura T, Sakaguchi R, Komukai S, Matsuyama T, et al. Association between early tracheostomy and patient outcomes in critically ill patients on mechanical ventilation: a multicenter cohort study. J Intensive Care 2022;10:19. ArticlePubMedPMCPDF
- 75. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181-247.PubMedPMC
Citations
Citations to this article as recorded by

 , Robert Alexander Jones-Baro1
, Robert Alexander Jones-Baro1 , Alberto Gómez-González1
, Alberto Gómez-González1 , Dalia Sahian Lugo-García1
, Dalia Sahian Lugo-García1 , Pía Carolina Gallardo Astorga1
, Pía Carolina Gallardo Astorga1 , Andrea Melo-Villalobos1
, Andrea Melo-Villalobos1 , Bárbara Kassandra Gonzalez-Rodriguez2
, Bárbara Kassandra Gonzalez-Rodriguez2 , Ángel Augusto Pérez-Calatayud3
, Ángel Augusto Pérez-Calatayud3








 KSCCM
KSCCM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite