Abstract
-
Background
- Although preoxygenation is an essential procedure for safe endotracheal intubation, in some cases securing sufficient time for tracheal intubation may not be possible. Patients with head and neck cancer might have a difficult airway and need a longer time for endotracheal intubation. We hypothesized that the extended apneic period with preoxygenation via a high-flow nasal cannula (HFNC) is beneficial to patients who undergo head and neck surgery compared with preoxygenation with a simple mask.
-
Methods
- The study was conducted as a single-center, single-blinded, prospective, randomized controlled trial. Patients were divided into groups based on one of the two preoxygenation methods: HFNC group or simple facemask (mask group). Preoxygenation was performed for 5 minutes with each method, and endotracheal intubation for all patients was performed using a video laryngoscope. Oxygen partial pressures of the arterial blood were compared at the predefined time points.
-
Results
- For the primary outcome, the mean arterial oxygen partial pressure (PaO2) immediately after intubation was 454.2 mm Hg (95% confidence interval [CI], 416.9–491.5 mm Hg) in the HFNC group and 370.7 mm Hg (95% CI, 333.7–407.4 mm Hg) in the mask group (P=0.002). The peak PaO2 at 5 minutes after preoxygenation was not statistically different between the groups (P=0.355).
-
Conclusions
- Preoxygenation with a HFNC extending to the apneic period before endotracheal intubation may be beneficial in patients with head and neck cancer.
-
Keywords: airway management; head and neck cancer; high-flow nasal cannula; preoxygenation
INTRODUCTION
Preoxygenation before endotracheal intubation promotes safety during intubation, and its importance has been taken for granted for many years [1]. However, in some cases securing sufficient time for endotracheal intubation despite conducting preoxygenation in patients may not be possible. For example, in patients with head and neck cancer, the cancer mass may obstruct the airway, or anatomical and/or pathological changes caused by previous surgery or radiation therapy that may cause difficulties in endotracheal intubation may be present. This may extend the time required for endotracheal intubation to longer than average duration and sometimes cause tracheal intubation to fail, even when performed by skilled specialists.
Although there have been several studies on the preoxygenation method using various methods [1-6], a simple face mask is the commonest tool. Unlike preoxygenation with a simple face mask, when preoxygenation using a high-flow nasal cannula (HFNC) is performed, the nasal cannula can be worn continuously during the induction of general anesthesia. Thus, apneic oxygenation is possible even in paralyzed and/or anesthetized patients [7-10]. Although the HFNC is a tool worth considering, especially when difficult airways are expected in patients, the effectiveness of a HFNC compared to the face mask in preoxygenating patients who are expected to have a difficult airway is unknown. Therefore, we hypothesized that preoxygenation using a HFNC can keep oxygen partial pressure higher during tracheal intubation than the simple face mask in patients who underwent head and neck surgery.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Asan Medical Center (No. 2019-0275) and was registered before patient enrolment at ClinicalTrials.gov (NCT03896906; April 1, 2019). Written informed consent was obtained from the participants. A thorough description of our study protocol has been published previously [11].
Study Population
The study involved patients with head and neck cancer who required reconstructive surgery using a free flap. The participants were between 19 and 80 years old. Only patients who were in good enough health to be categorized as American Society of Anesthesiologists physical status I–III patients were included in the study. Patients who did not wish to participate were not included.
Study Design and Blindness
The study was conducted as a single-center, assessor-blinded, prospective, randomized controlled trial. The participants were randomly assigned in a 1:1 ratio to the HFNC or simple mask group using a web-based program (http://www.randomization.com). Randomization was performed the day before surgery. Both the investigators and participants were blinded regarding group assignment until immediately before anesthesia administration; the grouping was revealed at that time due to the nature of the trial. To minimize potential bias, the statistician was blinded during the statistical analysis.
Standard monitoring, including non-invasive blood pressure, heart rate, oxygen saturation, and three-lead electrocardiogram, was performed; and arterial catheterization of the radial artery with a 20-G angiocatheter was conducted. After recording the baseline vital signs and arterial blood gas analysis (ABGA) data (T0), preoxygenation via the prepared method was initiated. Upper airway evaluation, including modified Mallampati score, inter-incisor gap, thyromental distances, and upper lip bite test, was performed before induction.
In the control group, preoxygenation was performed via an anesthetic facemask attached to the anesthesia apparatus without a reservoir bag (Supplementary Figure 1). 100% oxygen was provided at a flow rate high enough to prevent rebreathing (12 L/min), and the performer applied the mask tightly to prevent air leakage around the face mask to ensure adequate preoxygenation. During the preoxygenation period, subjects were asked to breathe naturally. For the HFNC group, 100% oxygen with a transnasal humidified oxygen delivery system (Optiflow THRIVE, Fisher and Paykel Healthcare) was supplied accordingly. Initially, oxygen was supplied at a flow rate of 30 L/min. Subsequently, the flow rate was gradually increased during the first 2 min; a maximum of 60 L/min was applied depending on the patient’s tolerance. The patients were asked to only breathe through their noses with their mouths closed.
The second and third data recordings were performed at 2 minutes (T1) and 5 minutes (T2), respectively, after the initiation of preoxygenation. Immediately after time T2, 40 mg of intravenous lidocaine and 1.5–2.0 mg/kg of propofol were administered; and 1.5 mg/kg of intravenous succinylcholine was injected sequentially. Endotracheal intubation was performed after onset of muscle relaxation, and the apnea time was estimated. The HFNC was maintained during the apneic period. However, in the simple mask group, the mask was removed to perform endotracheal intubation. The first intubation trial was conducted using a KoMAC Video Laryngoscope (KoMAC Co.). If the first attempt at intubation failed despite the use of video laryngoscope and cricoid compression, additional methods, such as the use of a bougie, video stylet, or fiberoptic bronchoscope, were applied. One or two instances of manual ventilation occurred in which the confirmation of endotracheal tube placement did not provide sufficient and regular tidal volume ventilation. We utilized gas analysis values measured during the second tidal cycle following the application of the ventilator preset tailored to the patient's needs immediately after confirming endotracheal tube placement. This allowed for more accurate measurement. The end-tidal partial pressure of oxygen (EtO2) and carbon dioxide (EtCO2) were measured via exhaled gas samples from the oral cavity and mask during the study period.
Outcome Assessment
The primary outcome was arterial oxygen partial pressure (PaO2) level immediately after endotracheal intubation (T3). The secondary outcomes were other data extracted from ABGA (PaCO2, pH, and SpO2) and hemodynamic data (heart rate and arterial blood pressure) at each time point. EtO2 and EtCO2 from exhaled gas at each time point were also used for analysis. Upper airway evaluation, including the modified Mallampati score, inter-incisor gap, thyromental distances, and upper lip bite test result, was performed before induction. After endotracheal intubation, we evaluated the intubation condition using factors such as the number of attempts at laryngoscopy, the use of any rescue airway maneuver, and the seniority of the anesthesiologist. Apnea time, defined as the period from propofol injection until the time the tracheal tube was secured, was recorded. Moreover, the satisfaction survey was conducted for the patients before their sedation and for the anesthesiologist after the induction of general anesthesia. The satisfaction level was categorized into four levels: very good, good, poor, and very poor.
Sample Size Calculation and Statistical Analysis
To calculate the sample size, we used G*Power 3.1.7 (Kiel University). Based on a previous report [5], 5 minutes of preoxygenation with HFNC achieved a median PaO2 of 406 mm Hg (Q2, 362; Q3, 446); and 5 minutes of face mask preoxygenation achieved a median PaO2 of 335 mm Hg (Q2, 292; Q3, 389). We estimated the mean (standard deviation) PaO2 with the HFNC and simple mask, 339 mm Hg (82.3) and 405 mm Hg (71.3), respectively. The sample size obtained using these values was 23 per group with alpha=0.05 and power=0.8 [12]. Estimating a screen failure of 10%, the appropriate sample size was 26 participants per group with a total of 52 participants.
To compare data between the groups, Student t-test or the Mann-Whitney U-test was used for continuous variables, and a chi-square test or Fisher’s exact test was used for categorical variables, as appropriate. To replace missing data resulting from the study period, a linear mixed-effect model was used to analyze and compare changes in continuous variables of ABGA from T0 to T3 within and between groups. All data manipulations and statistical analyses were performed using SPSS for Windows ver. 21 (IBM Corp.) and Stata software ver. 13.1 (StataCorp.). A two-tailed P-value of <0.05 was considered statistically significant.
RESULTS
Study Population and Intubation Conditions
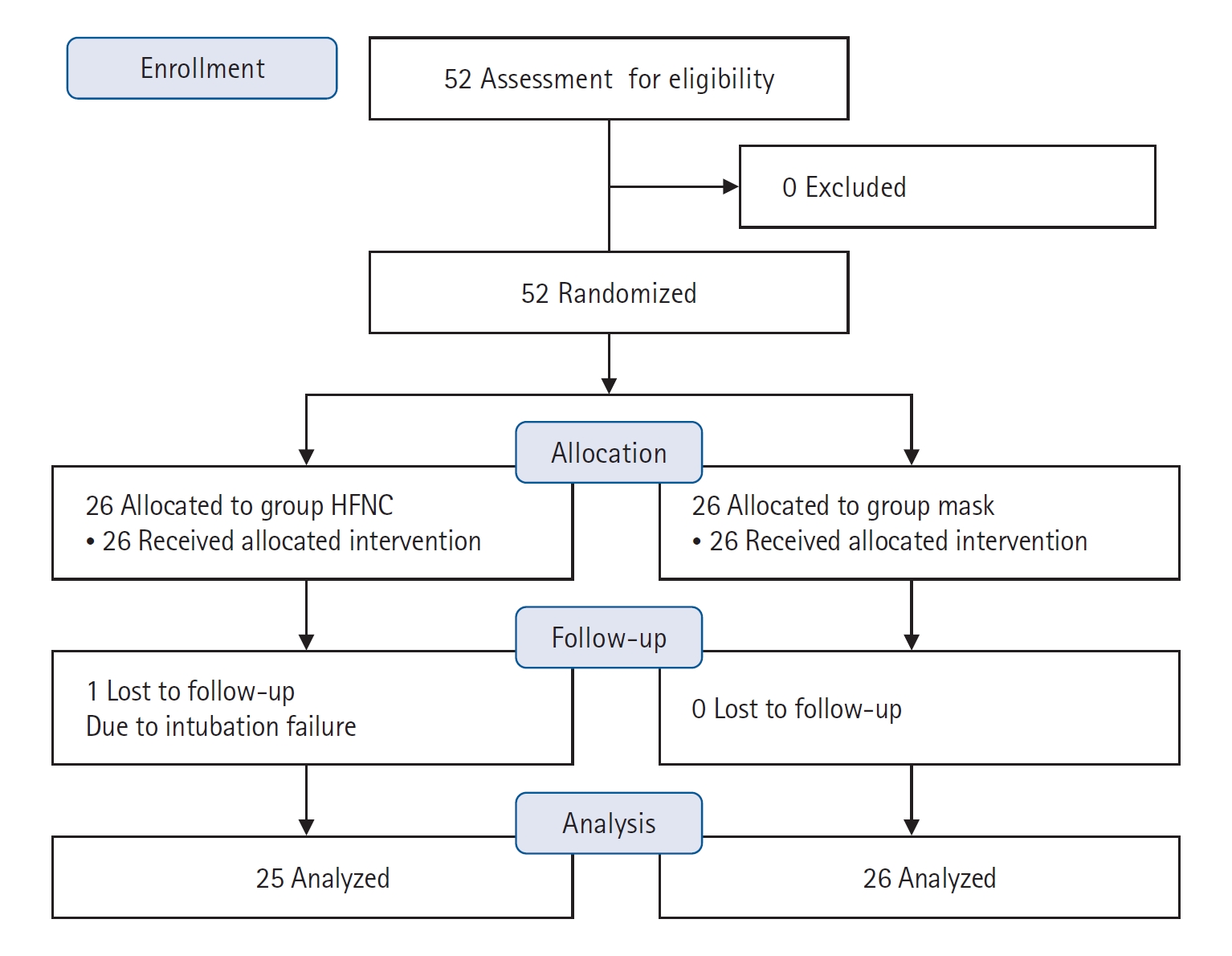
Fifty-two patients (26 in each group) in total were enrolled in the study, which was conducted from May 2019 to April 2020. One patient in the HFNC group was excluded due to intubation failure, and we analyzed data excluding this patient. The Consolidated Standards of Reporting Trials (CONSORT) diagram is provided in Figure 1. All patients were scheduled to undergo one of these surgeries with free flap reconstruction: craniofacial resection, total or partial glossectomy, total laryngectomy, mandibulectomy, maxillectomy, neck dissection, parotidectomy, or wide excision of tumors.
The basal demographic characteristics of the study population are shown in Table 1. The basal characteristics showed no difference between the two groups except for Mallampati classification. The signs predicting difficult intubation, which were Mallampati classifications, inter-incisor gap, thyromental distance, and upper lip bite test result, also showed no differences between the two groups (Table 1). As shown in Table 2, successful endotracheal intubation was achievable in most of the cases with or without additional maneuvers or additive instruments. All intubations were performed by senior residents or specialists who were experts in endotracheal intubation. The mean apnea times showed no significant difference between the two groups, 158.4±45.0 seconds and 152.2±72.3 seconds, in mask and HFNC groups, respectively (P=0.721). The longest safe apnea time was 432 seconds in the HFNC group. There was only one failed intubation in the HFNC group; the data from this patient was excluded in the data analysis for the primary outcome. In that case, mask ventilation was performed after T2 during emergent tracheostomy. The apnea time of the failed intubation case in the HFNC group was 810 seconds. In this failed-intubation case, the lowest SpO2 was 94%, and PaO2 was 248 mm Hg at T2. There was a statistically significant number of “good” or “very good” satisfaction ratings in the mask group (P=0.016), and patient satisfaction levels were significantly higher in the HFNC group (P=0.004). All patients except for one in the HFNC group had “good” and “very good” levels of satisfaction, while ten patients (38.4%) in the mask group complained of “poor” and “very poor” satisfaction.
Primary Outcome
PaO2 at T3, which was the primary outcome of this study, showed a statistically significant difference. PaO2 at T3 of the HFNC group was higher, 454.2 mm Hg (95% confidence interval [CI], 416.9–491.5 mm Hg), while that of the mask group was 370.7 mm Hg (95% CI, 333.7–407.4 mm Hg) (P=0.002). Oxygen saturation values measured via pulse oximeter at T3 were 100% except for one case in the HFNC group and three cases in the mask group. This difference was not statistically significant. The apnea time of the one case in the HFNC group was 200 seconds, and his PaO2 and SpO2 at T3 were 204 mm Hg and 99%, respectively. The lowest PaO2 (66 mm Hg) was recorded from a patient in the mask group, and the associated apnea time was 250 seconds.
Secondary Outcomes
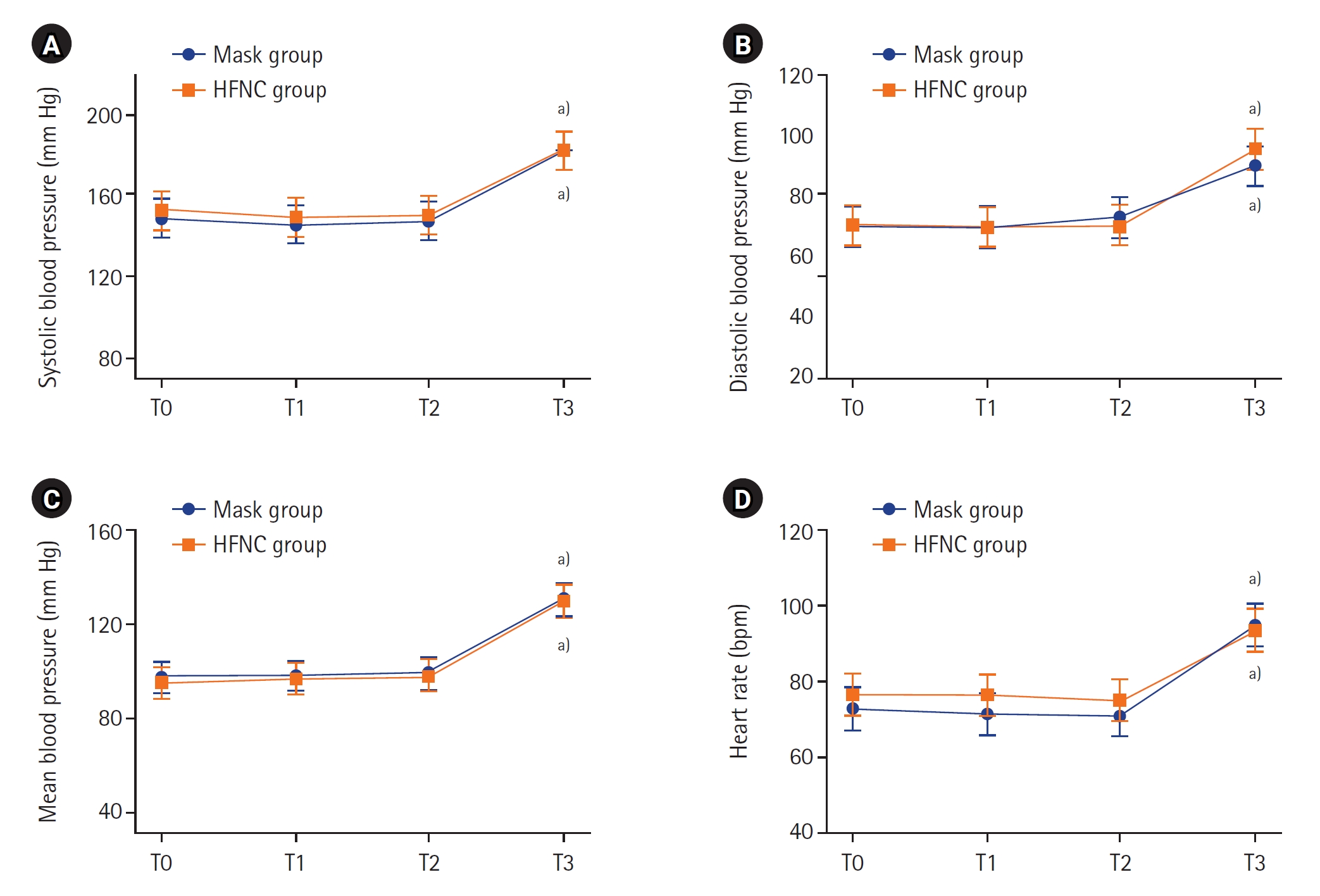
The changes in the gas analysis data from arterial blood and exhaled gas depending on each time point are shown in Figure 2 and Supplementary Figure 2, respectively. At the beginning of the study, PaO2 was similar in both groups before the patients reached a state of apnea. The peak PaO2 at T2 was not statistically different in either group (P=0.355) (Figure 2A, Supplementary Table 1). The PaCO2 of the HFNC group was lower than that of the mask group at all time points (Figure 2B). Supplementary Table 1 also shows that the differences of PaCO2 between the two groups were statistically significant (P-value of interaction effect=0.001). The pH changes of both groups are shown in Figure 2C. The P-value of both the group effect and time effect in both groups were 0.041, but the P-value of the interaction effect was 0.080. The patterns of change in EtCO2 and EtO2 were similar to the pattern of change in carbon dioxide and oxygen partial pressure in arterial blood (Supplementary Figure 2). However, the standard deviation was considerably large, and the difference between the two groups was not statistically significant. As shown in Figure 3 and Supplementary Table 2, all vital sign data at all time points were similar between the two groups.
DISCUSSION
Ever since the concept of preoxygenation before general anesthesia was introduced in clinical practice, oxygen supply with a simple face mask has been the conventional preoxygenation method prior to endotracheal intubation in general anesthesia. In this prospective randomized controlled study, preoxygenation using the HFNC showed higher PaO2 immediately after intubation compared to the conventional method with a simple face mask, even in cases of difficult airways. This result is consistent with those of other studies [5,13,14]. The remarkable point of the present study was that the study was conducted only on patients who had head and neck cancer that would obstruct their airways. Although the EtO2 is usually the most useful indicator of the completeness of preoxygenation [15], we used PaO2 as the primary parameter for preoxygenation. For the use of the HFNC, the EtO2 and EtCO2 measurements did not show reliable values since the inhaled and exhaled air was mixed with supplied high-flow oxygen as shown in the present study.
In the present study, the PaO2 in the HFNC group was higher than in the mask group immediately before the patients lost consciousness, the supposed time of full preoxygenation with self-respiration. This degree was maintained after intubation. HFNC has an incomparable advantage, the apneic oxygenation or the provision of oxygen during procedures such as endotracheal intubation [4,16]. This leads to longer safe apnea times in the HFNC group, allowing physicians to perform endotracheal intubation without hypoxia, even when the airway is difficult. In this study we were not able to compare the safe apnea times between the two groups because we did not delay endotracheal intubation until hypoxia developed.
A variable degree of preoxygenation depending on the patient is consistent with existing studies [2,13]. This was due to the patient’s tolerance of the rapid oxygen flow of HFNC and their cooperation in maintaining closed mouths and breathing only through their noses. There were several patients who could not withstand the rapid flow rate of 60 mL/min, and these patients were decelerated; thus, the oxygen flow applied to these patients before losing consciousness was reduced to 40–50 mL/min. However, in the context of the simple mask, efforts were made to increase the oxygen flow rate to 12 L/min to maximize the fraction of inspired oxygen (FiO2) delivery. However, due to the faster inspiratory flow, there is a possibility that FiO2 might not have reached 100%, potentially influencing the effectiveness of preoxygenation. Additionally, the presence of the Levin tube impeded the proper fitting of the mask, leading to varying degrees of preoxygenation among individuals.
The HFNC allows for CO2 removal even though that is not its main role. CO2 in patients in the mask group could only be eliminated when spontaneous ventilation was available. In contrast, PaCO2 did not increase significantly in patients in the HFNC group, even during apnea [17-21]. Rather, before T3 with spontaneous breathing, mild hypocarbia and acute repository alkalosis could be observed in the HFNC group. Eventually, the mask group accumulated more CO2 and induced more respiratory acidosis than the HFNC group at T3. Both acute respiratory acidosis and alkalosis could have a negative effect on the cardiovascular system and could lead to a crucial consequence [22-28]. However, the vital signs in both groups, including blood pressure and heart rate, showed no difference in the present study. This study demonstrates that lower pH and higher PaCO2 resulting from apneic periods do not play a critical role in vital signs in patients without severe cardiovascular or respiratory conditions. We found relatively low satisfaction among practitioners in the HFNC group, even though both hands could be free during preoxygenation. This might be due to lack of familiarity with HFNC. However, patient satisfaction levels were higher in the HFNC group. Patient discomfort was minimized since the oxygen flow rate with the HFNC was reduced to as much as the patients could tolerate. For those in the mask group with a Levin tube, the face mask had to be fitted as tightly as possible to seal patient airways for effective preoxygenation. This procedure and/or the tightness of the mask may have caused discomfort for the patients. In this study, a variety of difficult airway predictors were assessed in all subjects to demonstrate that difficult airways are common in patients with head and neck cancer. In fact, approximately 30% of the subjects in both groups were Mallampati class 3 or 4. Moreover, when the upper lip bite test was performed, more than 10% of patients were unable to bite their upper lips, suggesting a high prevalence of difficult airways in the test population [29].
The present study had several limitations. First, we could not blind the performers because the preoxygenation methods were markedly different in the study design. Second, in both groups the fraction of oxygen supplied to the patients could not be constantly maintained. Some patients in the mask group complained of discomfort and requested that the face mask not be applied too tightly. In addition, the oxygen delivery through the 12 L/min mask is slower than the inspiratory flow rate, so room air was forced to mix through the poorly fitted mask. Also, several patients in the HFNC group could not tolerate high oxygen flow rates, and the oxygen fraction supplied while the patients breathed through their mouths was also reduced in these cases. For both groups we had no choice but to proceed with the clinical trial within the patient's tolerance range. However, considering its implication for clinical practice, the results of this study, including those within the applicable range, are clinically significant. Last in this study, the preoxygenation method was divided into two types: mask or HFNC. If the HFNC is applied at a flow rate that the patient can tolerate before a simple face mask is applied simultaneously over the HFNC, more effective preoxygenation can be possible. Even the apneic oxygenation effect can be expected while performing endotracheal intubation with this method. This could be verified through further studies.
In conclusion, the preoxygenation via the HFNC showed higher pO2 immediately after intubation. The preoxygenation via the HFNC might be more beneficial to patients preparing to undergo endotracheal intubation compared to the conventional method of using a simple face mask as this may be associated with a longer and safer apneic time. Consistent with the results of previous studies, the HFNC is considered effective and useful even in airways prone to be difficult, especially those in patients with head and neck cancer.
KEY MESSAGES
▪ Preoxygenation before intubation is important for a safe procedure.
▪ Preoxygenation with the high-flow nasal cannula (HFNC) before intubation can safely extend the apneic period.
▪ The HFNC for preoxygenation may be beneficial in potentially difficult airways.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: JYJ, SSC. Data curation: JY, WJK, SK. Formal analysis: JYJ, JY, SSC. Methodology: JYJ, SSC. Project administration: SSC. Resources: JY, WJK, SK. Software: JYJ, SSC. Supervision: WJK, SK. Validation: SK. Visualization: JYJ. Writing–original draft: SK. Writing–review & editing: SSC, HJ.
Acknowledgments
We gratefully acknowledge the statistical advice of Seong-Sik Cho, MD, MPH, PhD (Department of Occupational and Environmental Medicine, Dong-A University College of Medicine, Busan, Korea).
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2022.01543.
Supplementary Figure 1.
The example of simple mask used in the study. We obtained the participant’s informed consent to use her photograph for research purposes and to publish generalized findings.
acc-2022-01543-Supplementary-Fig-1.pdf
Supplementary Figure 2.
Data from end-tidal gas analysis. Values are presented as estimated mean±95% confidence interval. P-values for interactions between group and time for end-tidal partial pressure of oxygen (EtO2; A) and end-tidal partial pressure of carbon dioxide (EtCO2; B) were 0.084 and 0.003, respectively. T0: preinduction baseline; T1: 2 minutes after preoxygenation; T2: 5 minutes after preoxygenation; T3: at the second tidal volume with mechanical ventilation after intubation; HFNC: high-flow nasal cannula.
acc-2022-01543-Supplementary-Fig-2.pdf
Figure 1.Consolidated Standards of Reporting Trials (CONSORT) flow diagram. HFNC: high-flow nasal cannula.
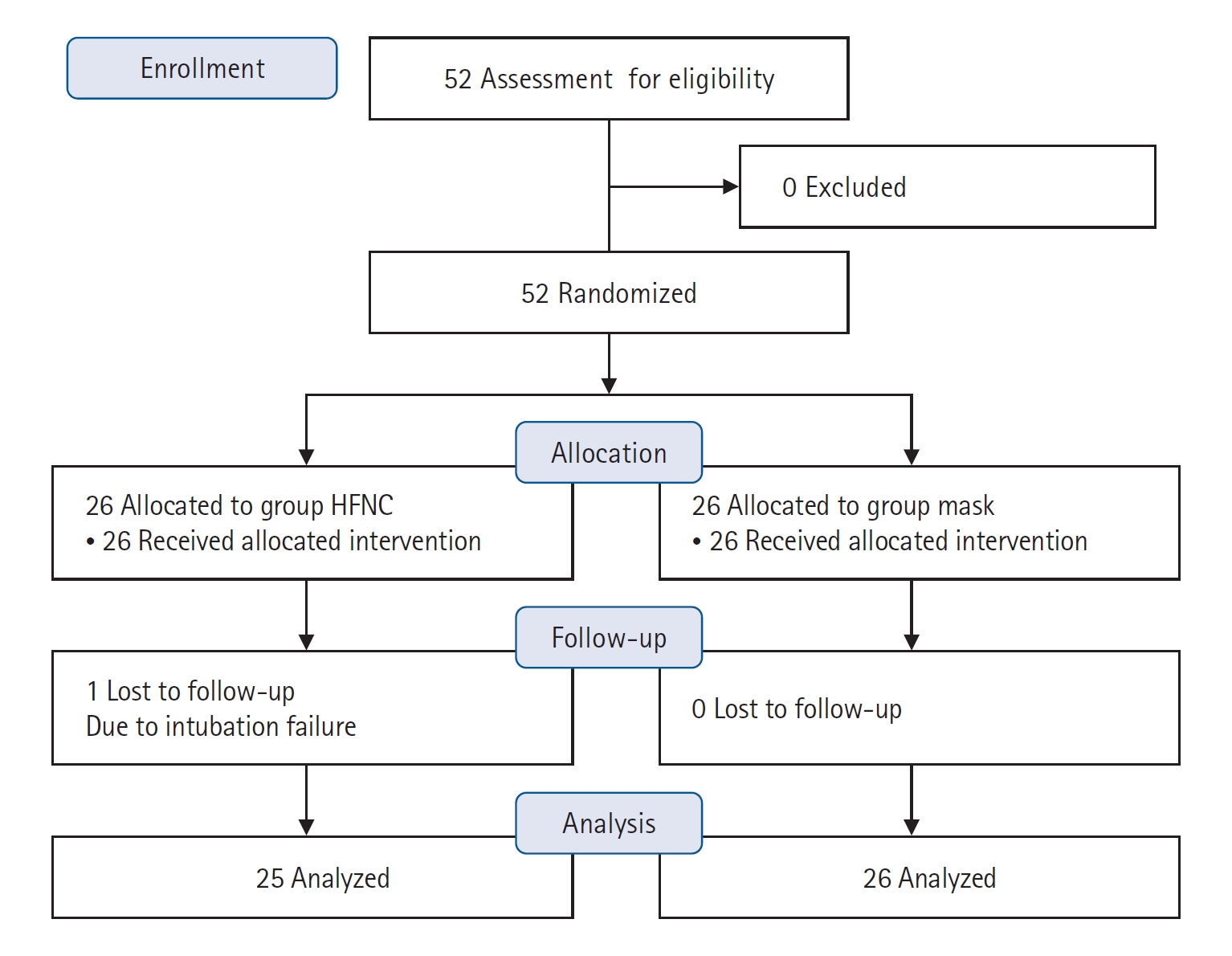
Figure 2.Data from arterial blood gas analysis. Values are presented as estimated mean±95% confidence interval. P-values for interactions between group and time for arterial oxygen partial pressure (PaO2; A), partial pressure of arterial carbon dioxide (PaCO2; B), and pH (C) were 0.008, 0.002, and 0.080, respectively. T0: preinduction baseline; T1: 2 minutes after preoxygenation; T2: 5 minutes after preoxygenation; T3: at the second tidal volume with mechanical ventilation after intubation; HFNC: high-flow nasal cannula. a) P<0.001 compared to baseline in each group; b) P<0.001 compared between groups.
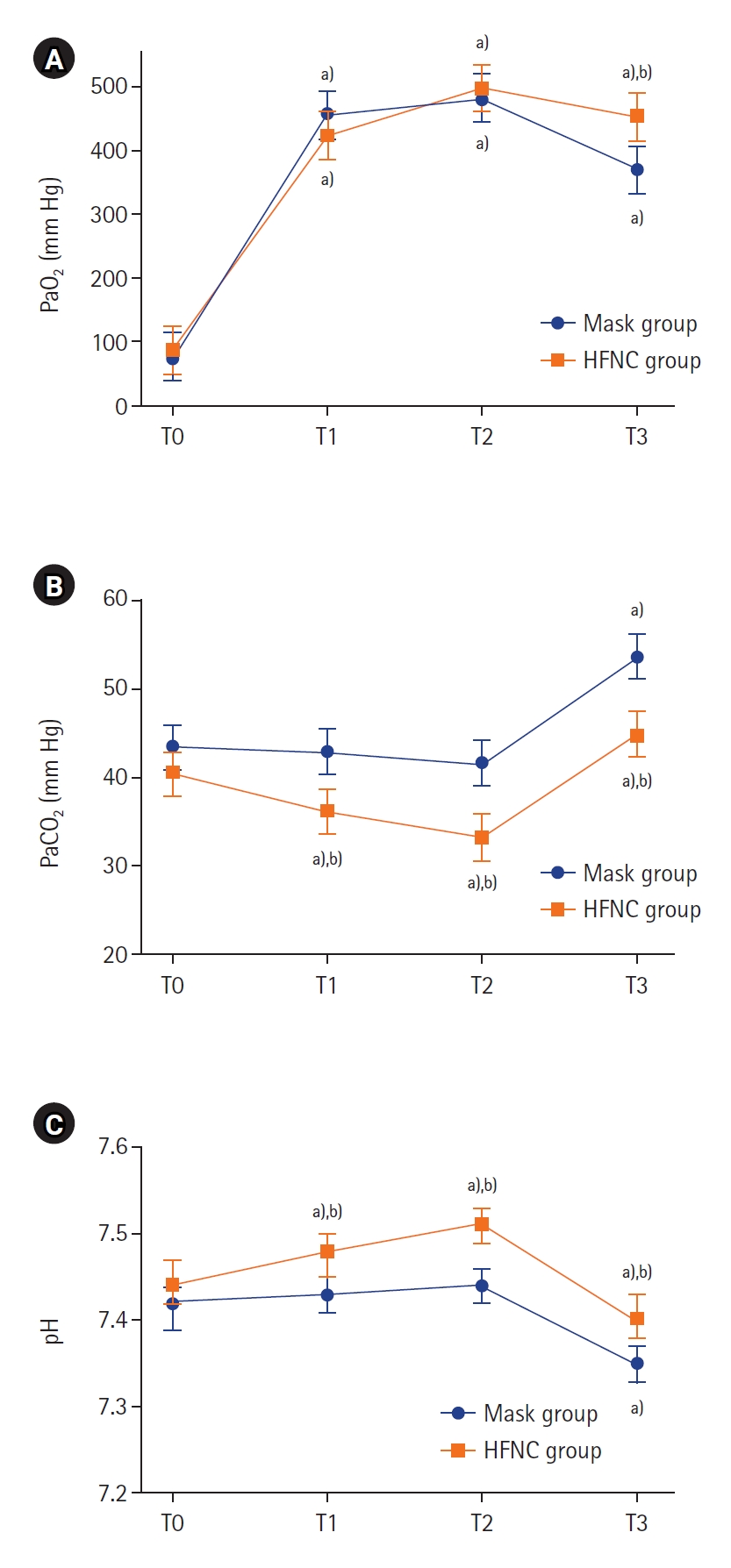
Figure 3.Changes in vital signs during induction. Values are presented as estimated mean±95% confidence interval. P-values for interactions between group and time for systolic blood pressure (A), diastolic blood pressure (B), mean blood pressure (C), and heart rate (D) were 0.955, 0.641, 0.995, and 0.112, respectively. T0: preinduction baseline; T1: 2 minutes after preoxygenation; T2: 5 minutes after preoxygenation; T3: at the second tidal volume with mechanical ventilation after intubation; HFNC: high-flow nasal cannula. a) P<0.001 compared to baseline in each group.
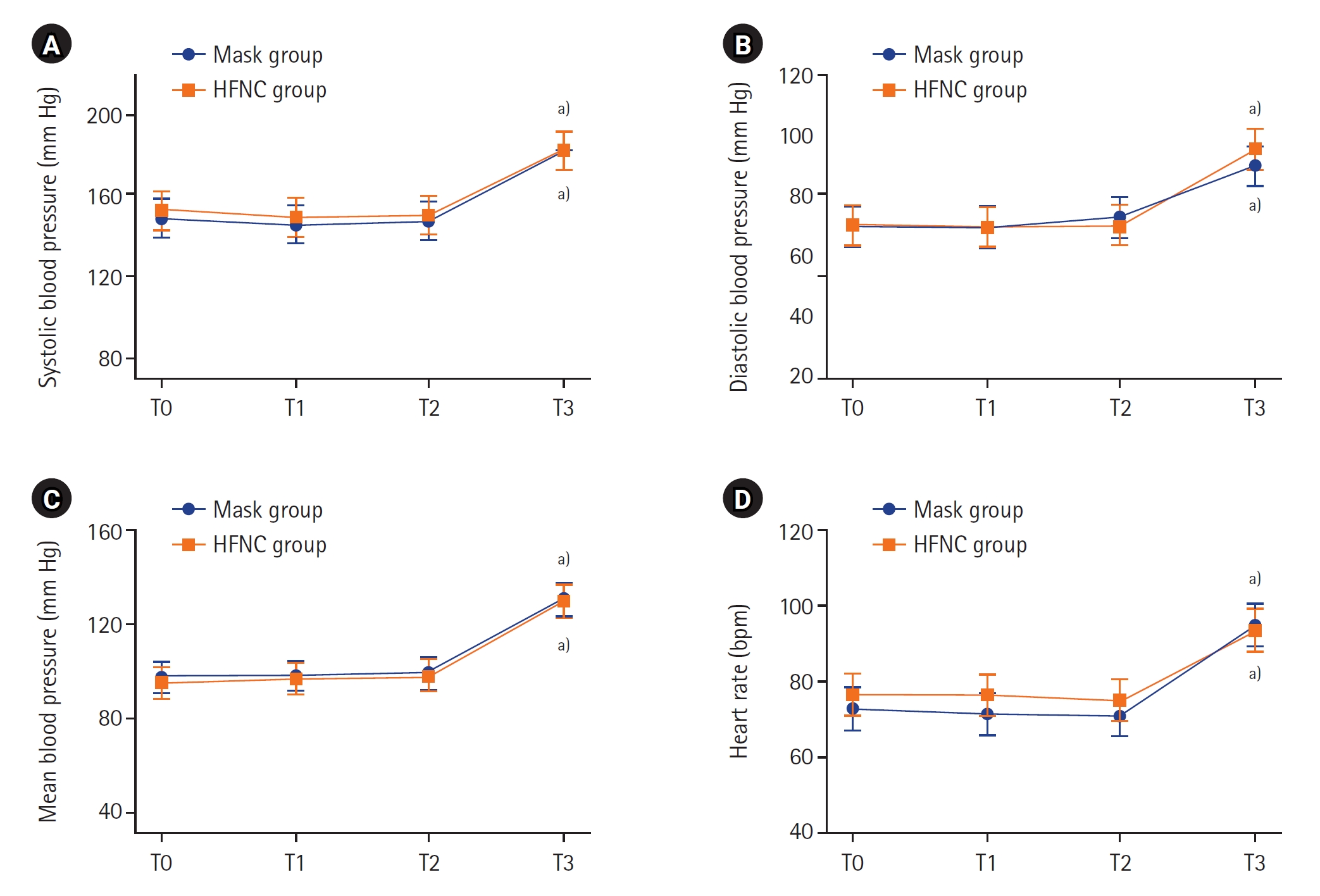
Table 1.Demographic data and airway assessments of the study population
|
Variable |
Preoxygenation group
|
P-value |
|
Mask (n=26) |
HFNC (n=25) |
|
Age (yr) |
61±12 |
56±17 |
0.211 |
|
Weight (kg) |
63.6±13.6 |
66.3±14.2 |
0.486 |
|
Height (cm) |
163.7±8.1 |
166.4±8.9 |
0.271 |
|
Body mass index (kg/m2) |
23.6±4.0 |
23.9±4.6 |
0.760 |
|
Female |
5 (19.2) |
5 (20.0) |
1.000 |
|
ASA physical status |
|
|
0.448 |
|
1 |
1 (3.8) |
3 (12.0) |
|
|
2 |
16 (61.5) |
12 (48.0) |
|
|
3 |
9 (34.6) |
10 (40.0) |
|
|
Comorbidity |
|
|
|
|
Hypertension |
6 (23.1) |
11 (44.0) |
0.144 |
|
Diabetes mellitus |
4 (15.4) |
5 (20.0) |
0.726 |
|
Pulmonary disease |
0 |
1 (4.0) |
0.490 |
|
Smoking |
|
|
0.691 |
|
Non-smoker |
12 (46.2) |
15 (60.0) |
|
|
Ex-smoker |
13 (50.0) |
9 (36.0) |
|
|
Current smoker |
1 (3.9) |
1 (4.0) |
|
|
Mallampati classification |
|
|
0.828 |
|
1 or 2 |
18 (69.2) |
18 (72.0) |
|
|
3 or 4 |
8 (30.8) |
7 (28.0) |
|
|
Interincisor gap (cm) |
4.2±1.2 |
4.1±1.1 |
0.823 |
|
Thyromental distance (cm) |
7.2±1.5 |
7.8±1.4 |
0.146 |
|
Upper lip bite test |
|
|
0.707 |
|
1 |
16 (61.5) |
14 (56.0) |
|
|
2 |
7 (26.9) |
4 (16.0) |
|
|
3 |
3 (11.5) |
4 (16.0) |
|
Table 2.Intubation conditions
|
Variable |
Preoxygenation group
|
Difference (95% CI) |
P-value |
|
Mask (n=26) |
HFNC (n=25) |
|
Intubation attempt |
|
|
|
|
|
1/2 or more |
25 (96.2)/1 (3.8) |
24 (96.0)/1 (4.0) |
0.2 (–10.5 to 10.8) |
0.999 |
|
Additional maneuver applieda)
|
7 (26.9) |
4 (16.0) |
–10.9 (–33.5 to 11.7) |
0.499 |
|
Apnea time (sec) |
158.4±45.0 |
152.2±72.3 |
–6.1 (–28.2 to 40.4) |
0.721 |
|
Intubation performer |
|
|
|
|
|
Resident/staff |
7 (26.9)/19 (73.1) |
10 (40.0)/15 (60.0) |
13.1 (–12.8 to 39.0) |
0.382 |
|
Performer’s satisfaction |
|
|
- |
0.016 |
|
Very good |
17 (65.4) |
7 (28.0) |
|
|
|
Good |
5 (19.2) |
9 (36.0) |
|
|
|
Poor |
2 (7.7) |
2 (8.0) |
|
|
|
Very poor |
0 |
4 (16.0) |
|
|
|
Not respondb)
|
2 (7.7) |
3 (12.0) |
|
|
|
Patient’s satisfaction |
|
|
- |
0.004 |
|
Very good |
8 (30.8) |
15 (60.0) |
|
|
|
Good |
6 (23.1) |
5 (20.0) |
|
|
|
Poor |
9 (34.6) |
0 |
|
|
|
Very poor |
1 (3.8) |
1 (4.0) |
|
|
|
Not respondb)
|
2 (7.7) |
4 (16.0) |
|
|
References
- 1. Bignami E, Saglietti F, Girombelli A, Briolini A, Bove T, Vetrugno L. Preoxygenation during induction of anesthesia in non-critically ill patients: a systematic review. J Clin Anesth 2019;52:85-90.ArticlePubMed
- 2. Shippam W, Preston R, Douglas J, Taylor J, Albert A, Chau A. High-flow nasal oxygen vs. standard flow-rate facemask pre-oxygenation in pregnant patients: a randomised physiological study. Anaesthesia 2019;74:450-6.ArticlePubMedPDF
- 3. Frat JP, Ricard JD, Quenot JP, Pichon N, Demoule A, Forel JM, et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Respir Med 2019;7:303-12.PubMed
- 4. Bouroche G, Bourgain JL. Preoxygenation and general anesthesia: a review. Minerva Anestesiol 2015;81:910-20.PubMed
- 5. Heinrich S, Horbach T, Stubner B, Prottengeier J, Irouschek A, Schmidt J. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J Obes Bariatrics 2014;1:7.
- 6. Azam Danish M. Preoxygenation and anesthesia: a detailed review. Cureus 2021;13:e13240.PubMedPMC
- 7. Mariyaselvam M, Stolady D, Wijewardena G, Blunt M, Young P. Transnasal humidified rapid insufflation ventilatory exchange for pre-oxygenation and apnoeic oxygenation during rapid sequence induction. Crit Care 2015;19(Suppl 1):P208. ArticlePMC
- 8. Stolady D, Mariyaselvam M, Young H, Fawzy E, Blunt M, Young P, et al. Pharyngeal oxygenation during apnoea following conventional pre-oxygenation and high-flow nasal oxygenation. Crit Care 2015;19(Suppl 1):P200. ArticlePMC
- 9. Grude O, Solli HJ, Andersen C, Oveland NP. Effect of nasal or nasopharyngeal apneic oxygenation on desaturation during induction of anesthesia and endotracheal intubation in the operating room: a narrative review of randomized controlled trials. J Clin Anesth 2018;51:1-7.ArticlePubMed
- 10. Ramachandran SK, Cosnowski A, Shanks A, Turner CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth 2010;22:164-8.ArticlePubMed
- 11. Jo JY, Kim WJ, Ku S, Choi SS. Comparison of preoxygenation with a high-flow nasal cannula and a simple mask before intubation during induction of general anesthesia in patients undergoing head and neck surgery: Study protocol clinical trial (SPIRIT Compliant). Medicine (Baltimore) 2020;99:e19525.PubMedPMC
- 12. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785-805.ArticlePubMedPDF
- 13. Pillai A, Daga V, Lewis J, Mahmoud M, Mushambi M, Bogod D. High-flow humidified nasal oxygenation vs. standard face mask oxygenation. Anaesthesia 2016;71:1280-3.ArticlePubMedPDF
- 14. Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia 2017;72:439-43.ArticlePubMedPDF
- 15. Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anaesth 2009;56:449-66.ArticlePubMedPDF
- 16. Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesth Analg 2017;124:507-17.ArticlePubMed
- 17. Lee HW, Choi SM, Lee J, Park YS, Lee CH, Yoo CG, et al. Reduction of PaCO2 by high-flow nasal cannula in acute hypercapnic respiratory failure patients receiving conventional oxygen therapy. Acute Crit Care 2019;34:202-11.ArticlePubMedPMCPDF
- 18. Kim HJ, Asai T. High-flow nasal oxygenation for anesthetic management. Korean J Anesthesiol 2019;72:527-47.ArticlePubMedPMCPDF
- 19. Kim ES, Lee H, Kim SJ, Park J, Lee YJ, Park JS, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis 2018;10:882-8.ArticlePubMedPMC
- 20. Huang Y, Lei W, Zhang W, Huang JA. High-flow nasal cannula in hypercapnic respiratory failure: a systematic review and meta-analysis. Can Respir J 2020;2020:7406457. ArticlePubMedPMCPDF
- 21. Lyons C, Callaghan M. Uses and mechanisms of apnoeic oxygenation: a narrative review. Anaesthesia 2019;74:497-507.ArticlePubMedPDF
- 22. Tournadre JP, Allaouchiche B, Malbert CH, Chassard D. Metabolic acidosis and respiratory acidosis impair gastro-pyloric motility in anesthetized pigs. Anesth Analg 2000;90:74-9.ArticlePubMed
- 23. Epstein SK, Singh N. Respiratory acidosis. Respir Care 2001;46:366-83.PubMed
- 24. Walley KR, Lewis TH, Wood LD. Acute respiratory acidosis decreases left ventricular contractility but increases cardiac output in dogs. Circ Res 1990;67:628-35.ArticlePubMed
- 25. Andersen MN, Mouritzen C. Effect of acute respiratory and metabolic acidosis on cardiac output and peripheral resistance. Ann Surg 1966;163:161-8.ArticlePubMedPMC
- 26. Fike CD, Hansen TN. The effect of alkalosis on hypoxia-induced pulmonary vasoconstriction in lungs of newborn rabbits. Pediatr Res 1989;25:383-8.ArticlePubMed
- 27. Morel J, Gergele L, Domine A, Molliex S, Perrot JL, Labeille B, et al. The venous-arterial difference in CO2 should be interpreted with caution in case of respiratory alkalosis in healthy volunteers. J Clin Monit Comput 2017;31:701-7.ArticlePubMedPDF
- 28. Samuelsson RG, Nagy G. Effects of respiratory alkalosis and acidosis on myocardial excitation. Acta Physiol Scand 1976;97:158-65.ArticlePubMed
- 29. Crawley SM, Dalton AJ. Predicting the difficult airway. BJA Educ 2015;15:253-7.Article
Citations
Citations to this article as recorded by

 , Jungpil Yoon
, Jungpil Yoon , Heeyoon Jang
, Heeyoon Jang , Wook-Jong Kim
, Wook-Jong Kim , Seungwoo Ku
, Seungwoo Ku , Seong-Soo Choi
, Seong-Soo Choi






 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite