Articles
- Page Path
- HOME > Acute Crit Care > Volume 30(3); 2015 > Article
- Case Report Septic Shock due to Unusual Pathogens, Comamonas testosteroni and Acinetobacter guillouiae in an Immune Competent Patient
- Hyun Jung Kim, M.D.1, Yunkyoung Lee, M.D.2, Kyunghwan Oh, M.D.2, Sang-Ho Choi, M.D., Ph.D.3, Heungsup Sung, M.D., Ph.D.4, Jin Won Huh, M.D., Ph.D.5
-
Korean Journal of Critical Care Medicine 2015;30(3):180-183.
DOI: https://doi.org/10.4266/kjccm.2015.30.3.180
Published online: August 30, 2015
1Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea
2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Department of Infectious Disease, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
4Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
5Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Correspondence to: Jin Won Huh, Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-3985, Fax: +82-2-3010-8039 E-mail: jwhuh@amc.seoul.kr
Copyright © 2015 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 21,466 Views
- 85 Download
- 6 Crossref
Abstract
- Comamonas testosteroni and Acinetobacter guillouiae are gram-negative bacilli of low virulence that are widely distributed in nature and normal flora. Despite their common occurrence in environments, they rarely cause infectious disease. We experienced a case of septic shock by C. testosterone and A. guillouiae, and isolated them by 16S ribosomal RNA sequencing method from the blood cultures of a previous healthy female during postoperative supportive care. This is the first case of septic shock required ventilator care and continuous renal replacement therapy due to these organisms in Korea.
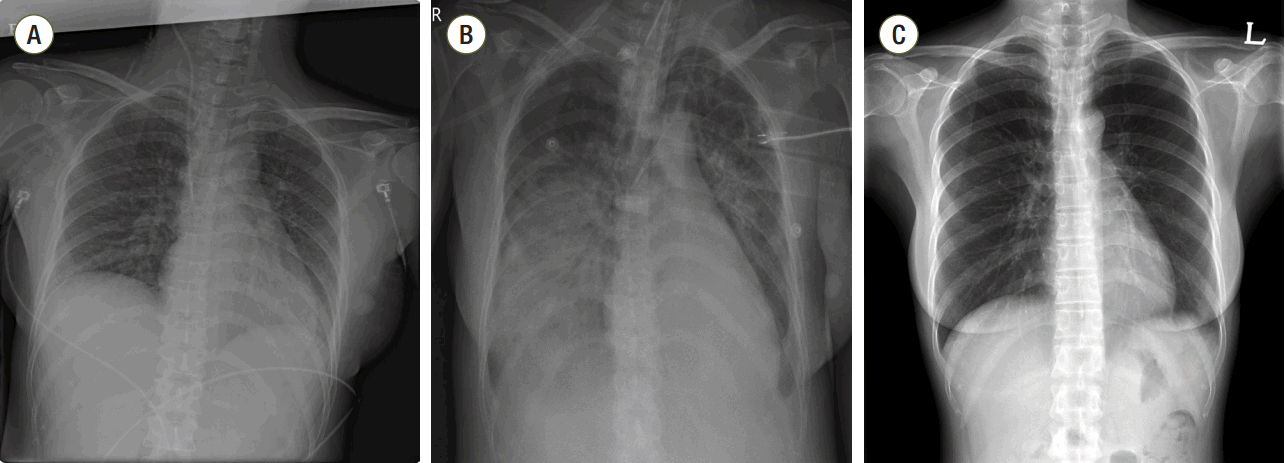
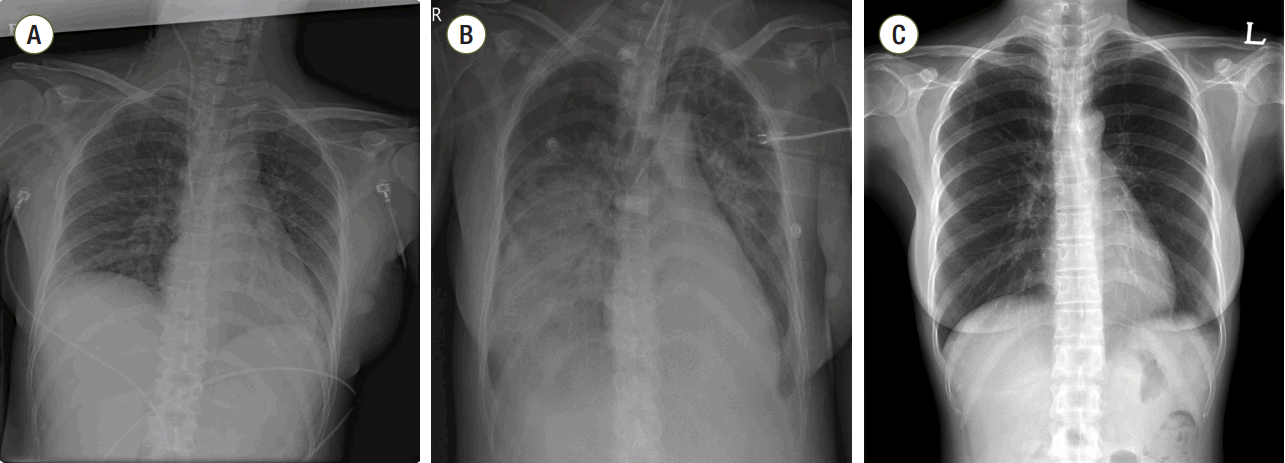
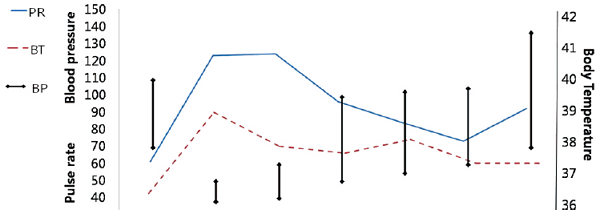
Case Report
Discussion
|
PR: pulse rate; BT: body temperature; BP: blood pressure; POD: postoperative day; ER: emergency room; HD: hospital day; CRRT: continuous renal replacement treatment; WBC: white blood cell; Hb: hemoglobin; PLT: platelet; CRP: c-reactive protein; PT: prothrombin time; AST: aspartate transaminase; ALT: alanine transaminase; Tbil: total bilirubin.
- 1. Farshad S, Norouzi F, Aminshahidi M, Heidari B, Alborzi A. Two cases of bacteremia due to an unusual pathogen, Comamonas testosteroni in Iran and a review literature. J Infect Dev Ctries 2012;6:521-5.ArticlePubMed
- 2. Tsui TL, Tsao SM, Liu KS, Chen TY, Wang YL, Teng YH, et al. Comamonas testosteroni infection in Taiwan: Reported two cases and literature review. J Microbiol Immunol Infect 2011;44:67-71.ArticlePubMed
- 3. Nseir W, Khateeb J, Awawdeh M, Ghali M. Catheter-related bacteremia caused by Comamonas testosteroni in a hemodialysis patient. Hemodial Int 2011;15:293-6.ArticlePubMed
- 4. Abraham JM, Simon GL. Comamonas testosteroni bacteremia: a case report and review of the literature. Infect Dis Clin Pract 2007;15:272-3.Article
- 5. Reddy AK, Murthy SI, Jalali S, Gopinathan U. Post-operative endophthalmitis due to an unusual pathogen, Comamonas testosteroni. J Med Microbiol 2009;58(Pt 3):374-5.ArticlePubMed
- 6. Nemec A, Musílek M, Sedo O, De Baere T, Maixnerová M, van der Reijden TJ, et al. Acinetobacter bereziniae sp. nov. and Acinetobacter guillouiae sp. nov., to accommodate Acinetobacter genomic species 10 and 11, respectively. Int J Syst Evol Microbiol 2010;60(Pt 4):896-903.ArticlePubMed
- 7. Singh NK, Khatri I, Subramanian S, Mayilraj S. Genome sequencing and annotation of Acinetobacter guillouii strain MTCC 11365T. Genomics Data 2014;2:4-6.ArticlePubMedPMC
- 8. Tayabali AF, Nguyen KC, Shwed PS, Crosthwait J, Coleman G, Seligy VL. Comparison of the virulence potential of Acinetobacter strains from clinical and environmental sources. PLoS One 2012;7:e37024.ArticlePubMedPMC
- 9. Kumar A, Kethireddy S, Darovic GO. Catheter-related and infusion-related sepsis. Crit Care Clin 2013;29:989-1015.ArticlePubMed
- 10. Gul M, Ciragil P, Bulbuloglu E, Aral M, Alkis S, Ezberci F. Comamonas testosteroni bacteremia in a patient with perforated acute appendicitis. Short communication. Acta Microbiol Immunol Hung 2007;54:317-21.ArticlePubMed
- 11. Bayhan GI, Tanir G, Karaman I, Ozkan S. Comamonas testosteroni: an unusual bacteria associated with acute appendicitis. Balkan Med J 2013;30:447-8.ArticlePubMedPMC
- 12. Janda JM, Abbott SL. 16S rRNA Gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 2007;45:2761-4.ArticlePubMedPMC
- 13. Wigand ME, Heidland A. Ototoxic side-effects of high doses of frusemide in patients with uraemia. Postgrad Med J 1971;47 Suppl:54-6.
- 14. Matz GJ. Aminoglycoside cochlear ototoxicity. Otolaryngol Clin North Am 1993;26:705-12.ArticlePubMed
References
Figure & Data
References
Citations

- An investigation of clinical characteristics and antimicrobial agent susceptibility patterns in clinical Comamonas testosteroni isolates: An increasingly prevalent nosocomial pathogen
Bahadır Orkun Ozbay, Adalet Aypak, Aliye Bastug, Ömer Aydos, İpek Mumcuoglu, Sevim Gayenur Büyükberber, Ayşe Müge Karcıoğlu, Hurrem Bodur
Infectious Diseases Now.2023; 53(2): 104622. CrossRef - Bullfrogs (Lithobates catesbeianus) as a Potential Source of Foodborne Disease
Andrea P. Zepeda-Velazquez, Fabián-Ricardo Gómez-De-Anda, Luis F. Aguilar-Mendoza, Nayeli Shantal Castrejón-Jiménez, Juan Carlos Hernández-González, Jorge A. Varela-Guerrero, Jorge-Luis de-la-Rosa-Arana, Vicente Vega-Sánchez, Nydia E. Reyes-Rodríguez
Journal of Food Protection.2023; 86(4): 100067. CrossRef - The Emergence of the Genus Comamonas as Important Opportunistic Pathogens
Michael P. Ryan, Ludmila Sevjahova, Rachel Gorman, Sandra White
Pathogens.2022; 11(9): 1032. CrossRef - A rare case of peritoneal dialysis‐associated peritonitis caused by Comamonas testosteroni
Roman Kuźniewicz, Mirosław Śnit, Dariusz Szczyra
Seminars in Dialysis.2022; 35(6): 556. CrossRef - The complex pattern of codon usage evolution in the family Comamonadaceae
Eugenio Jara, María A. Morel, Guillermo Lamolle, Susana Castro-Sowinski, Diego Simón, Andrés Iriarte, Héctor Musto
Ecological Genetics and Genomics.2018; 6: 1. CrossRef - First microbiota assessments of children's paddling pool waters evaluated using 16S rRNA gene-based metagenome analysis
Toko Sawabe, Wataru Suda, Kenshiro Ohshima, Masahira Hattori, Tomoo Sawabe
Journal of Infection and Public Health.2016; 9(3): 362. CrossRef

 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite