Abstract
-
Background
- Africa, like the rest of the world, has been impacted by the coronavirus disease 2019 (COVID-19) pandemic. However, only a few studies covering this subject in Africa have been published.
-
Methods
- We conducted a retrospective study of critically ill adult COVID-19 patients—all of whom had a confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection—admitted to the intensive care unit (ICU) of Habib Bourguiba University Hospital (Sfax, Tunisia).
-
Results
- A total of 96 patients were admitted into our ICU for respiratory distress due to COVID-19 infection. Mean age was 62.4±12.8 years and median age was 64 years. Mean arterial oxygen tension (PaO2)/fractional inspired oxygen (FiO2) ratio was 105±60 and ≤300 in all cases but one. Oxygen support was required for all patients (100%) and invasive mechanical ventilation for 38 (40%). Prone positioning was applied in 67 patients (70%). Within the study period, 47 of the 96 patients died (49%). Multivariate analysis showed that the factors associated with poor outcome were the development of acute renal failure (odds ratio [OR], 6.7; 95% confidence interval [CI], 1.75–25.9), the use of mechanical ventilation (OR, 5.8; 95% CI, 1.54–22.0), and serum cholinesterase (SChE) activity lower than 5,000 UI/L (OR, 5.0; 95% CI, 1.34–19).
-
Conclusions
- In this retrospective cohort study of critically ill patients admitted to the ICU in Sfax, Tunisia, for acute respiratory failure following COVID-19 infection, the mortality rate was high. The development of acute renal failure, the use of mechanical ventilation, and SChE activity lower than 5,000 UI/L were associated with a poor outcome.
-
Keywords: COVID-19; intensive care unit; prognosis; respiratory distress
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the coronavirus responsible for the coronavirus disease 2019 (COVID-19) pandemic that originated in China and spread rapidly worldwide. COVID-19 has affected 219 countries, with more than 118 million infected patients and more than 2.6 million deaths reported [1]. In Africa, more than 4 million cases have been reported, with more than 239,000 cases confirmed in Tunisia [1]. Cough and fever are the most prevalent symptoms, whereas gastrointestinal symptoms are relatively less common [2]. Severe cases of acute COVID-19 infection are generally characterized by acute respiratory distress, and the most frequently reported cause of death is refractory hypoxemia. In fact, it is well established that up to 5% of COVID-19 infected patients develop severe acute respiratory failure requiring intensive care unit (ICU) admission, as well as invasive and/or non-invasive mechanical ventilation (NIV) as supportive care. Moreover, the mortality rate reported worldwide for COVID-19 infected patients requiring ICU admission ranges from 17% to 62% [3].
In Africa, there is little information available about the clinical characteristics and prognosis of COVID‐19-infected patients [4-8]. Global mortality rates range from 4% to 5.6% [4-8]. In-hospital mortality is high, in particular for patients with acute respiratory distress requiring ICU admission and mechanical ventilation [4-8]. In fact, the mortality rate reported in severe cases ranges from 50% to 100% [4-8]. However, to the best of our knowledge, apart from some reported cases [9,10], there have been no studies from Tunisia concerning severe cases of patients with COVID-19 requiring ICU admission. We conducted this study to describe the clinical features and outcomes of severely ill patients with COVID-19 infection in Sfax, Tunisia.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Habib Bourguiba University Hospital (IRB No. 309/2021) and the requirement for written informed consent was waived by the ethics committee. This was a retrospective study of critically ill adult COVID-19 patients—all of whom had a confirmed SARS-CoV-2 infection—admitted between 01 September (the first case) and 04 December 2020 into the ICU of Habib Bourguiba University Hospital. In fact, in Sfax and during the first wave (March-May 2020) of the COVID-19 pandemic, the number of COVID-19 infected patients was very low (less than 60 patients) and all were admitted to the COVID department. During the second wave, severe COVID-19 cases characterized by acute respiratory distress were all hospitalized in the ICU of Habib Bourguiba University Hospital. Our COVID-19 ICU department is a 16-bed, medical-surgical ICU in a teaching hospital of 510 beds that serves as a first-line medical center for an urban population of more than one million inhabitants.
Case Definition
The diagnosis of COVID-19 infection was confirmed by reverse transcription polymerase chain reaction testing (kit: genesig real-time PCR CORONAVIRUS COVID-19; CE IVD; Catalogue no. Z-Path-COVID-19-CE). Tests were performed on nasopharyngeal swabs. We used chest computed tomography (CT) scan results in the early detection of COVID-19 pneumonia [11] when findings were classified by radiologists as being compatible with COVID-19. In fact, the presence of peripheral ground glass opacities associated with multilobe and posterior involvement, bilateral distribution, and subsegmental vessel enlargement (>3 mm), is compatible with the diagnosis of COVID-19 pneumonia in this specific pandemic situation. Chest CT scans were scored as having 0%, 25%, 50%, 75%, or 100% involvement [11].
Data Collection and Analysis
Data collected for hospitalized COVID-19 patients included epidemiological, clinical, laboratory, radiological, and treatment data on the day of admission and during ICU stay. Epidemiological and clinical data as well as the presence of pre‐existing medical conditions, initial symptoms and signs, and laboratory and radiographic findings on COVID-19 department admission and on COVID-19 ICU admission, time course of the acute illness, delay between initial symptoms and COVID-19 ICU department admission, treatment modalities, complications during hospitalization, and discharge status were also recorded. In our ICU, serum cholinesterase (SChE) activity and levels of other inflammatory biomarkers (C-reactive protein [CRP], procalcitonin [PCT], and leukocytes) were assessed on ICU admission, 72 hours after ICU admission, and as requested (if the patient developed fever and/or when a nosocomial infection was suspected). For each patient, we defined elevated levels of CRP, leukocytes and PCT as the highest value obtained during the ICU stay. Poor SChE activity was defined as the lowest recorded value during the ICU stay. SChE activity was measured in serum using a biochemistry analyzer (Cobas 6000 analyzer: module c501) with a spectrophotometric method. Normal values of SChE range from 5,320 to 12,290 UI/L. PCT was measured with an electrochemiluminescence method using a Cobas 6000 analyzer (e 601), and CRP was measured using an immunoturbidimetric method. All biomarker assays were run in the same laboratory.
In our ICU, the severity of illness was evaluated by the Simplified Acute Physiology Score (SAPS) II [12] and Sequential Organ Failure Assessment (SOFA) score [13]. Overweight and obesity were defined as a body mass index (BMI) ≥25 kg/m2 and BMI ≥30 kg/m2, respectively [14]. Acute respiratory distress syndrome (ARDS) was defined according to the recently published formal guidelines [15]. According to arterial oxygen tension (PaO2)/fractional inspired oxygen (FiO2) ratio, ARDS was classified as mild (200< PaO2/FiO2 ≤300 mm Hg), moderate (100< PaO2/FiO2 ≤200 mm Hg), or severe (PaO2/FiO2 ≤100 mmHg) [15]. FiO2 for patients able to spontaneously ventilate was calculated using the following formula: FiO2=(oxygen flow O2×4)+21%. For all patients, we assessed outcome variables including duration of mechanical ventilation, ICU and hospital length of stay, and ICU mortality.
Management of COVID-19 Patients with Respiratory Distress in Our COVID-19 ICU
In our practice, we used corticosteroids (dexamethasone, 12–24 mg/day) to treat patients with COVID-19 because of the variety of cytokines affected by this steroid (including interleukin [IL]-1, IL-6 and tumor necrosis factor α) [16]. Moreover, acute respiratory distress was treated via high-flow nasal oxygen or facial mask. However, non-invasive and/or invasive mechanical ventilation (IMV) as a supportive treatment was reserved for serious cases with severe hypoxemia. Prone position was also used to treat non-intubated COVID-19 patients and those in hypoxemic acute respiratory failure. For patients who needed IMV, we used a low tidal volume (<6 ml/kg ideal body weight), and airway pressure (plateau pressure <30 cmH2O) as recommended [17]. For patients with moderate/severe ARDS (PaO2/FiO2 ratio<150) requiring IMV, prone positioning was recommended for at least 18 hours per day. Vitamin C (3 g/day), azithromycin, zinc, diuretics (in the absence of shock and/or hypovolemia), and enoxaparin were used in all patients in the absence of contraindications.
Statistical Modeling
All statistical analyses were performed using the IBM SPSS ver. 22 (IBM Corp., Armonk, NY, USA). A P-value less than 0.05 was considered statistically significant. Continuous variables are reported as means (±standard deviation). Categorical variables are reported as proportions and percentages. For univariate analysis of categorical variables, we used the Pearson chi-square or Fisher exact test, as appropriate. The normality of the distribution of continuous variables was examined using the Shapiro-Wilk test. Student t-test was used to assess the significance of differences in normally distributed variables between groups, while the Mann-Whitney U-test was used for non-normally distributed variables. Next, a multivariate logistic regression analysis was performed by using a backward stepwise approach to identify factors predictive of death. Odds ratios and 95% confidence intervals were estimated from the β coefficients obtained. Some variables such as age and severity scores, SAPS II score, SChE activity, and other parameters were used to predict a poor outcome and were analyzed using receiver operating characteristic (ROC) curves. The area under the ROC curve, which was estimated by the method of Hanley and McNeill [18], provides a measure of the predictive accuracy of a test.
RESULTS
Descriptive Characteristics of All Included Patients at Admission
From September 1, 2020, to December 4, 2020, a total of 96 patients were admitted into our ICU for respiratory distress due to a COVID-19 infection. In this study, 77% of patients were male. Mean age was 62.4±12.8 years with a median of 64 years (Table 1), and 41 patients (43%) had a BMI above 30 kg/m2. Mean SAPS II on ICU admission was 33±16 (median, 29) and the mean SOFA score on ICU admission was 6±3 (median, 4). Moreover, 78 patients (81%) had one or more comorbidities. The most common pre-existing medical conditions were arterial hypertension in 48 patients (50%) and diabetes mellitus in 44 (46%). The most common symptoms at admission to the hospital were dyspnea in 88 patients (92%), fever (≥38°C) in 63 (66%), and cough in 59 (62%). Extra-pulmonary symptoms, such as headache, were observed in 23 patients (24%), and anosmia/ageusia was observed in 15 patients (15.6%). Mean time elapsed from onset of symptoms to admission was 6.5±4.2 days, and mean time elapsed from symptom onset to ICU admission was 10.7±5.6 days. The most frequent causes of ICU admission were acute respiratory failure (n=89 patients, 92.7%) and coma (5, 5.4%) (Table 1 and 2). On ICU admission, clinical examination showed that all patients exhibited major signs of respiratory distress. Mean respiratory rate was 29±12 breaths per minute, mean oxygen saturation measured by pulse oximetry (SpO2) was 87%±13% under oxygen support via facial mask and 63%±20% in room air. Mean body temperature was at 37°C±0.8°C, and only 12 patients had a body temperature above 38°C. Moreover, 38 patients (41%) showed signs of increased respiratory effort (such as retractions, accessory muscle use, sweating, etc.), and 14 patients (14.6%) developed circulatory failure requiring the infusion of a vasopressor (noradrenaline) to maintain a mean arterial pressure of 65 mm Hg or greater. The mean Glasgow coma scale (GCS) score on ICU admission was at 13±3. Ten patients (10.4%) were deeply comatose with a GCS of less than 8. Table 1 summarizes the characteristics of the entire population group on hospital and ICU admission.
Biological and Radiological Findings
Mean pH on ICU admission was 7.40±0.08 with a range of 7.13–7.58. Mean PaO2/FiO2 ratio was 105±60, and was ≤ 300 in all cases but one (Table 3). For patients who needed IMV, mean PaO2/FiO2 ratio was 69±22, <150 in all patients, and ≤100 (severe ARDS) in 90% of patients. Metabolic acidosis (pH <7.38 and bicarbonate [HCO3–] <22 mmol/L) was observed in 12 patients (14%). Mean blood sugar level on admission was 12.9±6.3 mmol/L (ranging from 3.3 to 32 mmol/L); a high blood sugar level (8 mmol/L) was observed in 78% of cases. Mean plasma protein concentration was 69±8.1 g/L. Mean blood urea was 12.2±9.3 mmol/L, and was >10 mmol/L in 48% of cases. Mean blood creatinine was 107±98 µmol/L, and > 100 µmol/L in 25% of cases. Among the inflammatory parameters recorded at admission for patients with available data, mean leukocyte number was 13,220±6,014 cells/mm3, and >11,000/mm3 in 63% of cases. Mean lymphocyte number was 713±432 cells/mm3, and lymphopenia (≤1,000 cells/mm3) was observed in 79.5% of cases. Mean CRP concentration was 134±152 mg/L, and ≥100 mg/L in 46% of cases. Mean PCT was 1.7±6.1 ng/mL, ranging from 0.1 to 46 ng/mL. SChE activity was measured for 78 patients on ICU admission (median number of measurements during ICU stay was 2), and mean SChE activity was 5,857±1,773 UI/L (range, 1,926–10,190 UI/L). Finally, the mean D-dimer value was 1,359±1,430μg/L. Table 3 summarizes all the biological findings of the entire patient population on ICU admission. At hospital and/or ICU admission, CT was performed in 75 patients (78%), and findings were compatible with COVID-19 in 75 of 75 of cases (100%). Chest CT scans showed parenchymal opacification with 25% to less than 50% involvement in 14 patients (18.6%), 50% to less than 75% involvement in 31 patients (41%), and 75% or greater involvement in 30 patients (40%). Four patients (4.2%) were diagnosed with pulmonary embolism.
Treatment and Clinical Outcomes
During ICU stay, 78 patients (81.0%) received antibiotics, even though only four patients (4.1%) had a confirmed bacterial coinfection. Antibiotics received were azithromycin (n=78, 81%), beta-lactams (n=39, 40%), and levofloxacin (n=25, 26%), and 28 patients (29%) received other antibiotics for nosocomial infections. In addition, corticosteroids were administered to 94 patients (98%). Prophylactic dose anticoagulation for venous thromboembolism was used in 10 patients (10.4%), and therapeutic-dose anticoagulation was used in 86 patients (90%). Vitamin C (3 g/day) was prescribed for all patients, neuromuscular blockers for 38 patients (39.5%), and diuretics for 71 patients (74%). Oxygen support was required for all patients (100%) and was administered via facial mask for 75 (78%), high-flow nasal oxygen for 29 (30.2%), NIV for 48 (50%), and IMV for 38 patients (40%). Among patients requiring IMV, 10 patients were previously treated by high-flow nasal oxygen and 26 previously managed with NIV.
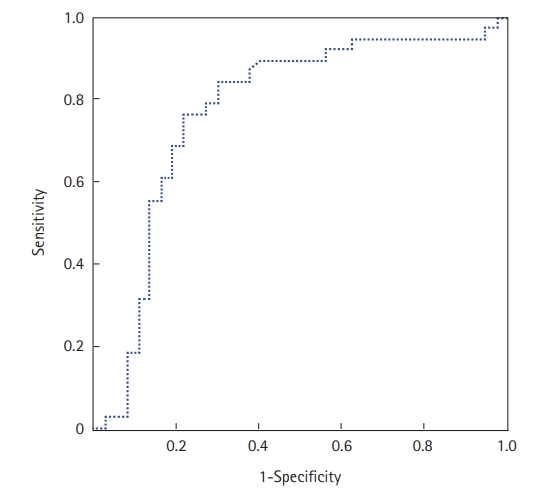
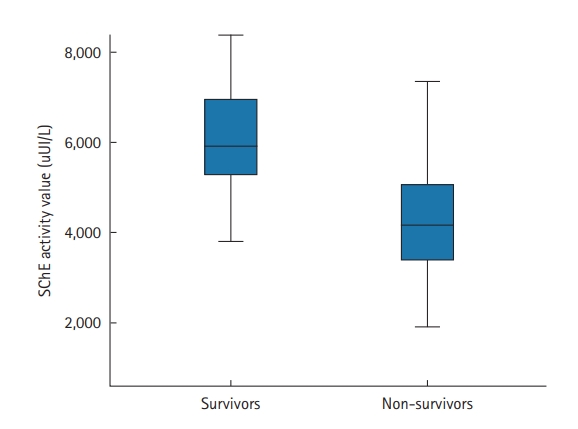
The prone position was applied in 67 patients (70%); it was utilized to treat 29 COVID-19 patients who were not intubated (50%) and 38 patients who were intubated (100%). Extracorporeal membrane oxygenation was used in one patient (1.6%), and 36 (37.5%) patients required vasopressors. Within the study period, 47 of the 96 included patients died (49%). The mean ICU stay was 6.7±5.3 days (median, 5 days). Factors associated with death in univariate analyses were antecedent arterial hypertension, severe respiratory distress, and the presence of neurological impairment. Table 4 shows all the factors associated with death in univariate analysis. In our study, SChE activity greater than 5,000 UI/L during ICU stay was associated with a good outcome with 76% sensitivity and 79% specificity and an area under the curve (AUC) of 0.77 (Figure 1). Figure 2 shows the lowest SChE activity value (poor value) recorded in all patients during ICU stay according to outcomes. A CRP value greater than 180 mg/L was associated with a poor outcome with a sensitivity of 60% and specificity of 79% with an AUC of 0.73 (Figure 3).
Finally, multivariate analysis showed that factors associated with poor outcome were the development of acute renal failure (ARF; OR, 6.7), the use of mechanical ventilation (OR, 5.8), and SChE activity lower than 5,000 UI/L (OR, 5.0) (Table 5).
DISCUSSION
Principal Findings
Our study confirms that severe cases of COVID-19 are characterized by acute respiratory distress, and the most common cause of death is refractory hypoxemia. Within the study period, 47 of our 96 patients died (49%). Despite the non-use of specific treatments (antiviral agents) [19] in our ICU, the mortality rate of 49% was similar to those reported in other studies [15-18,20,21], particularly in developed countries [22-24].
Management of Severe COVID-19 Infection
In our ICU, we use corticosteroids (dexamethasone, 12–24 mg/day), which are known for their ability to regulate a number of cytokines, to treat SARS-CoV-2-infected patients [16]. Acute respiratory distress was treated via high-flow nasal oxygen or facial mask. Nonetheless, severe cases in urgent need of supportive care, namely those suffering from acute hypoxemia, had to be placed under invasive and/or NIV. As recommended [19,25], zinc, vitamin D and vitamin C, diuretics (in the absence of hypovolemia and/or shock), and enoxaparin were administered to all patients if there were no contraindications. In fact, there is some evidence that deficiency of one or more of these three elements (zinc, vitamin D, vitamin C) compromises the immune response, making an individual more vulnerable to viral infections and to a worse disease prognosis [25]. We did not use hydroxychloroquine to treat patients admitted to our ICU following severe respiratory distress due to COVID-19 based on the results of many studies [20,21,26] and the World Health Organization. However, we used the prone position to treat COVID-19 patients who were not intubated and those with severe respiratory failure and hypoxemia. In fact, it has been established that the timely use of the prone position in patients with a COVID-19 infection suffering from severe hypoxemia can improve oxygenation and prevent the need for IMV, ultimately decreasing the number of COVID-19 patients who die due to complications of severe hypoxemia [27].
Clinical Outcomes
The high mortality rate (49%) observed in our study, although somewhat similar to those reported in other studies [15-18], particularly in developed countries [22-24], can be explained by several factors. First, 78 patients (81%) had one or more comorbidities with a median age of 64 years. Moreover, on ICU admission, clinical examination showed that all patients exhibited major signs of respiratory distress and chest CT scans showed parenchymal opacification with more than 50% involvement in more than 81% of cases. Moreover, 36 patients (37.5%) required vasopressors during their ICU stay. In the current study, we found that the development of ARF was associated with a poor outcome.
Our study confirms the results of several previous studies [28,29] that reported that the development of ARF was strongly associated with severe clinical outcomes and mortality. In fact, patients with COVID-19 with ARF had a 3-fold higher odds of death than COVID-19 patients without ARF, and a 4-fold higher odds of death than ARF due to other causes [30]. Moreover, we found in the current study that SChE activity lower than 5,000 UI/L was associated with a poor outcome in multivariate analysis. Our results confirm the results of a recent study performed by Nakajima et al. [31] who found that cholinesterase level on admission was an independent predictor of the severity of COVID-19 pneumonia and mortality. In fact, cholinesterase activity is thought to be affected by acute-phase infections and inflammatory processes [32]. Cholinesterase inhibition can be caused by inflammatory mediators (cytokines) released in severe cases of COVID-19 pneumonia. In the current study, only 28 patients (29%) developed nosocomial infections. Despite the lower rate of nosocomial infections reported (40.7%) in an earlier study [33], it is within the range reported in other studies (15%–40%) [34,35].
Study Limitations
Our study had several limitations. First, its retrospective nature is a methodological limitation, as all retrospective studies suffer from incomplete information. In our study, CRP and PCT levels and SChE activity were not assessed in all patients admitted to the ICU. Moreover, SChE activity was not measured at the same time point in all patients. In addition, because of a lack of equipment, extracorporeal membrane oxygenation was used in only one patient among 38 mechanically ventilated patients. Finally, we did not follow-up patients after ICU discharge. However, this study is one of the first to study the clinical manifestations, management, and outcomes of patients with severe COVID-19 infection requiring ICU admission in Africa.
In this retrospective cohort study of critically ill patients admitted to the ICU in Sfax, Tunisia, for acute respiratory failure following COVID-19 infection, the mortality rate was high. The development of ARF, the use of mechanical ventilation, and SChE activity lower than 5,000 UI/L were associated with poor outcomes. Further studies are needed on this subject.
KEY MESSAGES
▪ In this retrospective cohort study of critically ill patients admitted to an intensive care unit in Sfax, Tunisia, for acute respiratory failure following coronavirus disease 2019 (COVID-19) infection, the mortality rate was high (49%).
▪ The development of acute renal failure, the use of mechanical ventilation, and serum cholinesterase activity lower than 5,000 UI/L were associated with poor outcomes.
NOTES
-
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conceptualization: MB (Mabrouk Bahloul). Data curation: OT, MB, SK, KC, RA, NK, MH, HC, HK, MB. Formal analysis: MB, OCW, CBH, KBM, AK, NR, MB. Methodology: MB (Mabrouk Bahloul). Writing–original draft: OT, MB, MH, NB, RA, NK, MH, OCW, CBH, HC, KBM, AK, HK, NR, MB. Writing–review & editing: MB, SK, KC, MH, OCW, MB.
Figure 1.Receiver operating characteristic curve of serum cholinesterase (SChE) activity to predict mortality. The area under the curve was 0.77, indicating good ability of SChE to discriminate between survivors and non-survivors.
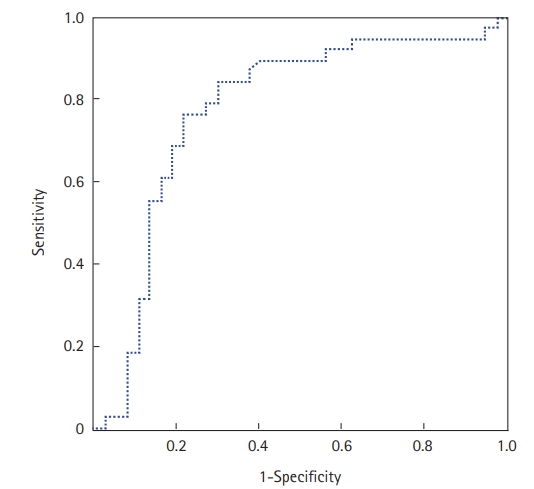
Figure 2.The lowest serum cholinesterase (SChE) activity value recorded according to outcomes. Black line, median; box, 25%–75%; error bar, range.
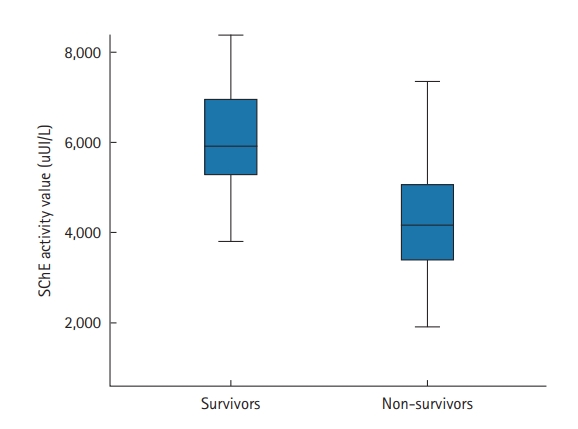
Figure 3.Receiver operating characteristic curve of C-reactive protein level to predict mortality. The area under the curve was 0.73.
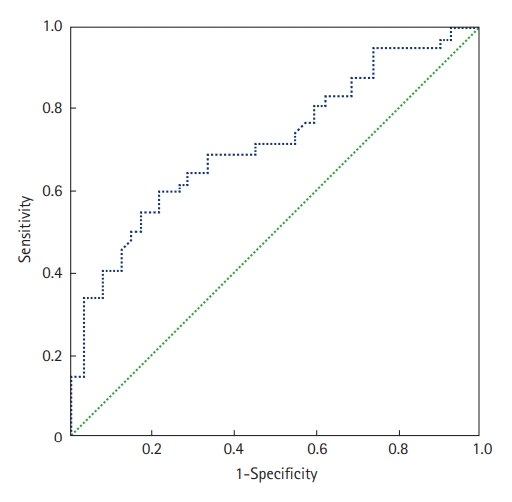
Table 1.Clinical presentation of the study subjects on hospital admission and on the day of ICU admission
|
Variable |
Value |
|
Demographic characteristics |
|
|
Age (yr) |
62.4±12.8 |
|
Obesity (BMI >30 kg/m2) |
41 (43) |
|
Diabetes mellitus |
44 (46) |
|
Arterial hypertension |
48 (50) |
|
Chronic heart disease |
8 (8) |
|
Chronic obstructive pulmonary disease |
5 (5) |
|
Symptom on hospital admission |
|
|
Dyspnea |
88 (92) |
|
Cough |
59 (62) |
|
Fever |
63 (66) |
|
Headache |
23 (24) |
|
Anosmia/ageusia |
15 (16) |
|
Vomiting |
13 (14) |
|
Abdominal pain |
15 (16) |
|
Diarrhea |
8 (8) |
Table 2.The clinical presentation the day of ICU admission
|
Variable |
Value |
|
SAPS II score |
33±16 |
|
SOFA score |
6±3 |
|
Cause of ICU admission |
|
|
Respiratory distress |
89 (93) |
|
Coma |
5 (5) |
|
Shock |
1 (1) |
|
Clinical finding on ICU admission |
|
|
Respiratory rate/min |
29±12 |
|
SpO2 under O2 (%) |
87±13 |
|
Signs of respiratory exertiona
|
38 (41) |
|
Shock |
14 (15) |
|
Body temperature (°C) |
37±08 |
|
Treatment |
|
|
Sedation |
48 (50) |
|
Vasopressor support |
36 (38) |
|
High-flow nasal oxygen |
29 (31) |
|
NIV |
48 (50) |
|
IMV |
38 (40) |
|
Prone positioning |
67 (70) |
|
Corticosteroids |
94 (98) |
|
Diuretics |
71 (74) |
Table 3.Laboratory findings of the entire study group on ICU admission
|
Parameter |
Data available for |
Mean±SD |
Median (range) |
|
WBC count (cells/mm3) |
88 |
13,220.8±6,014.1 |
12,000 (1,120–31,000) |
|
Lymphocyte (cells/mm3) |
70 |
713.6±432.5 |
600 (100–1,800) |
|
Hemoglobin (g/dl) |
88 |
12.5±2.1 |
13 (5.5–17.7) |
|
Platelet count (cells/mm3) |
88 |
303,298±121,920 |
288,000 (82,000–656,000) |
|
Blood glucose level (mmol/L) |
93 |
12.9±6.3 |
11 (3.3–32.1) |
|
AST (UI/L) |
86 |
41.7±32.7 |
32 (9.9–204) |
|
ALT (UI/L) |
86 |
34.1±23.8 |
24 (7–120.5) |
|
Total bilirubin (μmol/L) |
78 |
9.4±4.9 |
8 (4–29) |
|
Total protein (g/L) |
66 |
69.3±8.13 |
70 (43–85) |
|
Prothrombin ratio (%) |
68 |
76±16.7 |
78 (29–100) |
|
Troponin (ng/ml) |
79 |
0.59±0.11 |
0.018 (0.001–0.64) |
|
Pro-BNP (pg/ml) |
60 |
2,289.9±5,502.3 |
403.5 (13–27,067) |
|
pH |
86 |
7.40±0.08 |
7.42 (7.13–7.58) |
|
PaCO2 (mm Hg) |
86 |
36.4±9.4 |
36 (15.9–62) |
|
PaO2 (mm Hg) |
86 |
74.4±28.4 |
68 (37–160) |
|
HCO3– (mmol/L) |
86 |
23.1±4.7 |
23 (8.2–38) |
|
PaO2/FiO2 ratio |
86 |
105.7±60.8 |
86 (37–338) |
|
Urea (mmol/L) |
91 |
12.3±9.3 |
9.7 (1–59.6) |
|
Creatinine (μmol/L) |
92 |
107±98.5 |
79.5 (37–759) |
|
Iron (μmol/L) |
60 |
10±6.6 |
7.7 (3.4–32) |
|
Ferritin (μg/L) |
56 |
1,063.1±638.9 |
922 (159–2,000) |
|
Procalcitonin (µg/L) |
65 |
1.53±5.89 |
0.25 (0.01–46.0) |
|
C-reactive protein (mg/L) |
64 |
134±152 |
92.5 (7–413) |
|
SChE activity (UI/L) |
78 |
5,857±1,773 |
5,794 (1,926–10,190) |
Table 4.Factors associated with death in univariate analysis
|
Risk factor |
Non-survivor (n=47) |
Survivor (n=49) |
P-value |
|
Age (yr) |
65.5±12.3 |
59.3±12.6 |
0.016 |
|
Arterial hypertension (comorbidity) |
29 (62) |
19 (38.8) |
0.025 |
|
Decompensated diabetes |
23 (24) |
12 (12.5) |
0.008 |
|
Shock on ICU admission |
12 (12.5) |
2 (2.1) |
0.003 |
|
Invasive mechanical ventilation |
35 (36.5) |
3 (3.1) |
<0.001 |
|
Noninvasive ventilation |
36 (37.5) |
12 (12.5) |
<0.001 |
|
Bacterial nosocomial infection |
26 (27.1) |
2 (2.1) |
<0.001 |
|
Kidney failure |
37 (38.5) |
14 (14.6) |
<0.001 |
|
Hypernatremia |
10 (10.4) |
1 (1) |
0.002 |
|
Hyperkalemia |
14 (14.6) |
2 (2.1) |
<0.001 |
|
SpO2 under air room (without O2) |
55±19 |
70±18 |
0.001 |
|
Respiratory rate (breaths/min) |
33±15 |
27±9 |
0.026 |
|
Heart rate (beats/min) |
97±22 |
85±19 |
0.008 |
|
Glasgow coma scale |
12.5±4.2 |
14.5±1.8 |
0.003 |
|
SOFA score |
6±3.5 |
3.8±1.9 |
<0.001 |
|
SAPS II score |
39.4±18.3 |
27.9±11 |
<0.001 |
|
White blood cell (cells/mm3) |
14,627±6,393 |
11,814±5,315 |
0.027 |
|
Urea (mmol/L) |
15±12.2 |
9.8±4.5 |
0.011 |
|
Creatinine (µmol/L) |
129.5±134.1 |
86.5±32.5 |
0.046 |
|
SChE activity (UI/L) on ICU admission |
5,471±1,723 |
6,242±1,763 |
0.082 |
|
CRP (mg/L) on ICU admission |
166±196 |
104±86 |
0.074 |
|
Lowest SChE activity (UI/L) |
4,574±1,821 |
6,059±1,628 |
<0.001 |
|
Highest CRP value (mg/L) |
224±129.5 |
127.5±92.5 |
<0.001 |
|
pH |
7.31±0.09 |
7.43±0.04 |
<0.001 |
|
PaO2/FiO2 ratio on ICU admission |
92.2±44.8 |
117.1±70.2 |
0.020 |
|
Lowest PaO2/FiO2 ratio |
68.8±25.9 |
97.2±59.9 |
0.008 |
|
Lowest HCO3– (mmol/L) |
19.3±5.8 |
21.5±4.0 |
0.049 |
Table 5.Independent variables associated with a poor outcome
|
Variable |
P-value |
Odds ratio (95% CI) |
|
Development of acute renal failure |
0.006 |
6.7 (1.75–25.9) |
|
Use of mechanical ventilation |
0.009 |
5.8 (1.54–22) |
|
SChE activity lower than 5,000 UI/L |
0.016 |
5.0 (1.34–19) |
References
- 1. Worldometer. Coronavirus update [Internet]. Worldometer. 2020;[cited 2020 Jul 24]. Available from: https://www.worldometers.info/coronavirus/.
- 2. Vetter P, Vu DL, L'Huillier AG, Schibler M, Kaiser L, Jacquerioz F. Clinical features of covid-19. BMJ 2020;369:m1470. ArticlePubMed
- 3. Chaddha U, Kaul V, Agrawal A. What is the true mortality in the critically ill patients with COVID-19? Indian J Crit Care Med 2020;24:383-4.ArticlePubMedPMC
- 4. Olumade TJ, Uzairue LI. Clinical characteristics of 4499 COVID-19 patients in Africa: a meta-analysis. J Med Virol 2021;93:3055-61.ArticlePubMedPMC
- 5. Abayomi A, Odukoya O, Osibogun A, Wright O, Adebayo B, Balogun M, et al. Presenting symptoms and predictors of poor outcomes among 2,184 patients with COVID-19 in Lagos State, Nigeria. Int J Infect Dis 2021;102:226-32.ArticlePubMed
- 6. El Vally A, Bollahi MA, Ould Ahmedou Salem MS, Deida J, Parola P, Basco L, et al. Retrospective overview of a COVID-19 outbreak in Mauritania. New Microbes New Infect 2020;38:100788. ArticlePubMedPMC
- 7. Ibrahim OR, Suleiman BM, Abdullahi SB, Oloyede T, Sanda A, Gbadamosi MS, et al. Epidemiology of COVID-19 and predictors of outcome in Nigeria: a single-center study. Am J Trop Med Hyg 2020;103:2376-81.ArticlePubMedPMC
- 8. Matangila JR, Nyembu RK, Telo GM, Ngoy CD, Sakobo TM, Massolo JM, et al. Clinical characteristics of COVID-19 patients hospitalized at Clinique Ngaliema, a public hospital in Kinshasa, in the Democratic Republic of Congo: a retrospective cohort study. PLoS One 2020;15:e0244272.ArticlePubMedPMC
- 9. Bahloul M, Ketata W, Lahyeni D, Mayoufi H, Kotti A, Smaoui F, et al. Pulmonary capillary leak syndrome following COVID-19 virus infection. J Med Virol 2021;93:94-6.ArticlePubMed
- 10. Saida IB, Ennouri E, Nachi R, Meddeb K, Mahmoud J, Thabet N, et al. Very severe COVID-19 in the critically ill in Tunisia. Pan Afr Med J 2020;35(Suppl 2):136. Article
- 11. Hare SS, Rodrigues JCL, Nair A, Jacob J, Upile S, Johnstone A, et al. The continuing evolution of COVID-19 imaging pathways in the UK: a British Society of Thoracic Imaging expert reference group update. Clin Radiol 2020;75:399-404.ArticlePubMedPMC
- 12. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63.ArticlePubMed
- 13. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754-8.ArticlePubMed
- 14. World Health Organization. Obesity [Internet]. Geneva, World Health Organization. 2020;[cited 2020 Jul 24]. Available from: https://www.who.int/topics/obesity/en/.
- 15. Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019;9:69. ArticlePubMedPMC
- 16. RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693-704.ArticlePubMed
- 17. Makkar P, Pastores SM. Respiratory management of adult patients with acute respiratory distress syndrome due to COVID-19. Respirology 2020;25:1133-5.ArticlePubMed
- 18. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839-43.ArticlePubMed
- 19. Lo Bianco G, Di Pietro S, Mazzuca E, et al. Multidisciplinary approach to the diagnosis and in-hospital management of COVID-19 infection: a narrative review. Front Pharmacol 2020;11:572168. ArticlePubMedPMC
- 20. Agarwal N, Biswas B, Lohani P. Epidemiological determinants of COVID-19 infection and mortality: a study among patients presenting with severe acute respiratory illness during the pandemic in Bihar, India. Niger Postgrad Med J 2020;27:293-301.ArticlePubMed
- 21. Brown WA, Moore EM, Watters DA. Mortality of patients with COVID-19 who undergo an elective or emergency surgical procedure: a systematic review and meta-analysis. ANZ J Surg 2021;91:33-41.ArticlePubMed
- 22. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020;180:1345-55.PubMed
- 23. Verma AA, Hora T, Jung HY, Fralick M, Malecki SL, Lapointe-Shaw L, et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ 2021;193:E410-8.ArticlePubMedPMC
- 24. Roedl K, Jarczak D, Thasler L, Bachmann M, Schulte F, Bein B, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Aust Crit Care 2021;34:167-75.ArticlePubMed
- 25. Name JJ, Souza AC, Vasconcelos AR, Prado PS, Pereira CP. Zinc, vitamin D and vitamin C: perspectives for COVID-19 with a focus on physical tissue barrier integrity. Front Nutr 2020;7:606398. ArticlePubMedPMC
- 26. Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med 2020;17:e1003501.ArticlePubMedPMC
- 27. Zang X, Wang Q, Zhou H, Liu S, Xue X, COVID-19 Early Prone Position Study Group. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med 2020;46:1927-9.ArticlePubMedPMC
- 28. Kang SH, Kim SW, Kim AY, Cho KH, Park JW, Do JY. Association between chronic kidney disease or acute kidney injury and clinical outcomes in COVID-19 patients. J Korean Med Sci 2020;35:e434.ArticlePubMedPMC
- 29. Fabrizi F, Alfieri CM, Cerutti R, Lunghi G, Messa P. COVID-19 and acute kidney injury: a systematic review and meta-analysis. Pathogens 2020;9:1052. ArticlePubMedPMC
- 30. Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med 2020;17:e1003406.ArticlePubMedPMC
- 31. Nakajima K, Abe T, Saji R, Ogawa F, Taniguchi H, Yamaguchi K, et al. Serum cholinesterase associated with COVID-19 pneumonia severity and mortality. J Infect 2021;82:282-327.Article
- 32. Bahloul M, Baccouch N, Chtara K, Turki M, Turki O, Hamida CB, et al. Value of serum cholinesterase activity in the diagnosis of septic shock due to bacterial infections. J Intensive Care Med 2017;32:346-52.ArticlePubMed
- 33. Bardi T, Pintado V, Gomez-Rojo M, Escudero-Sanchez R, Azzam Lopez A, Diez-Remesal Y, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis 2021;40:495-502.ArticlePubMedPMC
- 34. Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One 2021;16:e0251170.ArticlePubMedPMC
- 35. Bassetti M, Kollef MH, Timsit JF. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med 2020;46:2071-4.ArticlePubMedPMC
Citations
Citations to this article as recorded by

- Cost Effectiveness of Strategies for Caring for Critically Ill Patients with COVID-19 in Tanzania
Hiral Anil Shah, Tim Baker, Carl Otto Schell, August Kuwawenaruwa, Khamis Awadh, Karima Khalid, Angela Kairu, Vincent Were, Edwine Barasa, Peter Baker, Lorna Guinness
PharmacoEconomics - Open.2023; 7(4): 537. CrossRef - Prognostic Value of Serum Cholinesterase Activity in Severe SARS-CoV-2–Infected Patients Requiring Intensive Care Unit Admission
Mabrouk Bahloul, Sana Kharrat, Saba Makni, Najeh Baccouche, Rania Ammar, Aida Eleuch, Lamia Berrajah, Amel Chtourou, Olfa Turki, Chokri Ben Hamida, Hedi Chelly, Kamilia Chtara, Fatma Ayedi, Mounir Bouaziz
The American Journal of Tropical Medicine and Hygiene.2022; 107(3): 534. CrossRef
 , Sana Kharrat1, Kamilia Chtara1, Malek Hafdhi1, Olfa Turki1, Najeh Baccouche1, Rania Ammar1, Nozha Kallel2, Majdi Hsairi3, Olfa Chakroun-Walha4, Chokri Ben Hamida1, Hedi Chelly1, Khaiereddine Ben Mahfoudh2, Abelhamid Karoui3, Hela Karray5, Noureddine Rekik4, Mounir Bouaziz1
, Sana Kharrat1, Kamilia Chtara1, Malek Hafdhi1, Olfa Turki1, Najeh Baccouche1, Rania Ammar1, Nozha Kallel2, Majdi Hsairi3, Olfa Chakroun-Walha4, Chokri Ben Hamida1, Hedi Chelly1, Khaiereddine Ben Mahfoudh2, Abelhamid Karoui3, Hela Karray5, Noureddine Rekik4, Mounir Bouaziz1 









 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite