Articles
- Page Path
- HOME > Acute Crit Care > Volume 38(4); 2023 > Article
-
Review Article
Trauma Abdominal compartment syndrome in critically ill patients -
Hyunseok Jang1
 , Naa Lee1
, Naa Lee1 , Euisung Jeong1
, Euisung Jeong1 , Yunchul Park1
, Yunchul Park1 , Younggoun Jo1
, Younggoun Jo1 , Jungchul Kim1
, Jungchul Kim1 , Dowan Kim2
, Dowan Kim2
-
Acute and Critical Care 2023;38(4):399-408.
DOI: https://doi.org/10.4266/acc.2023.01263
Published online: November 29, 2023
1Division of Trauma, Department of Surgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
2Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- Corresponding author: Dowan Kim Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital, Chonnam National University Medical School, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea Tel: +82-62-220-6543, Fax: +82-6227-1636, E-mail: maskjoa@naver.com
Copyright © 2023 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,735 Views
- 2,086 Download
- Abstract
- INTRODUCTION
- PATHOPHYSIOLOGY
- DIAGNOSIS
- MEASUREMENTS
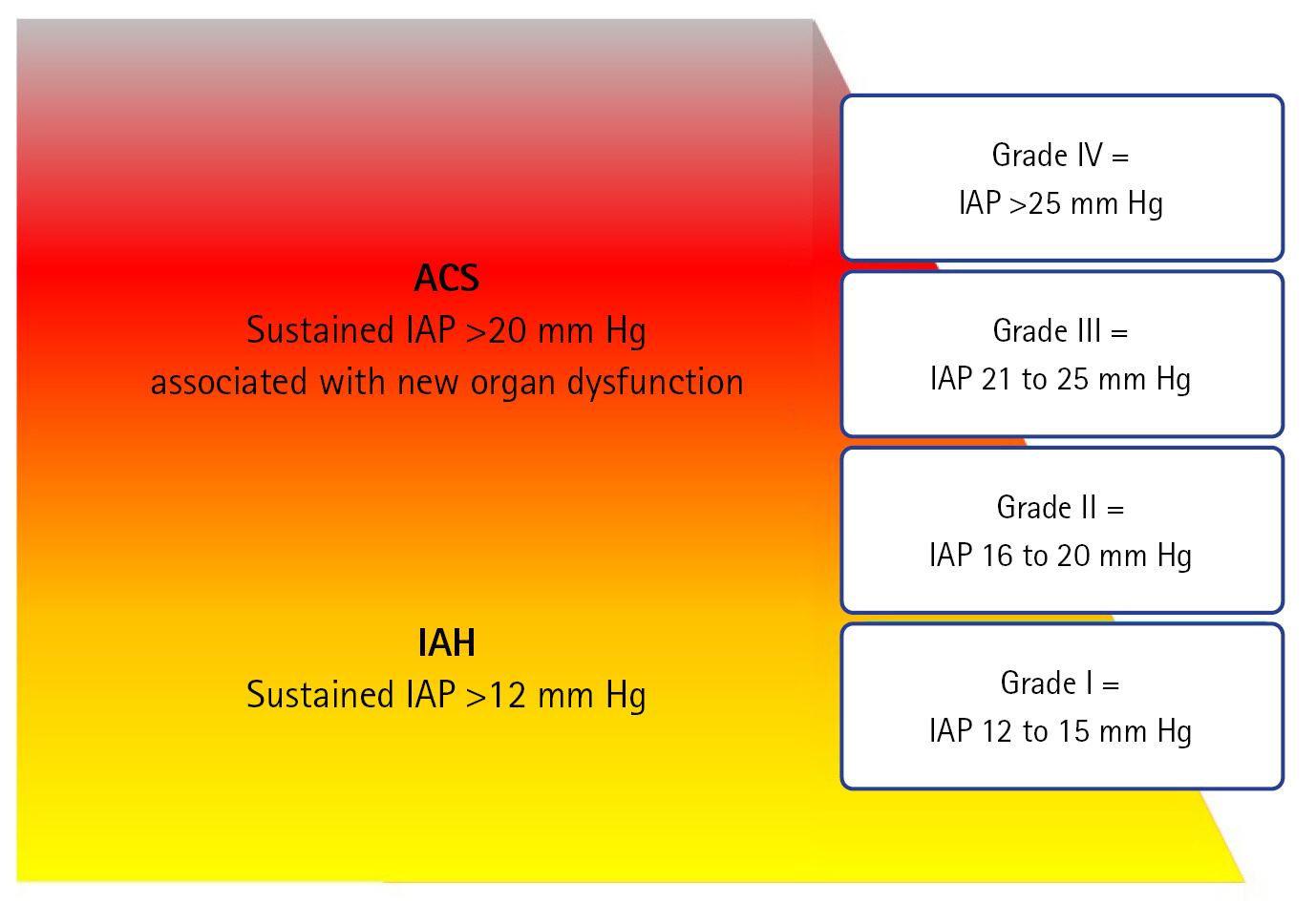
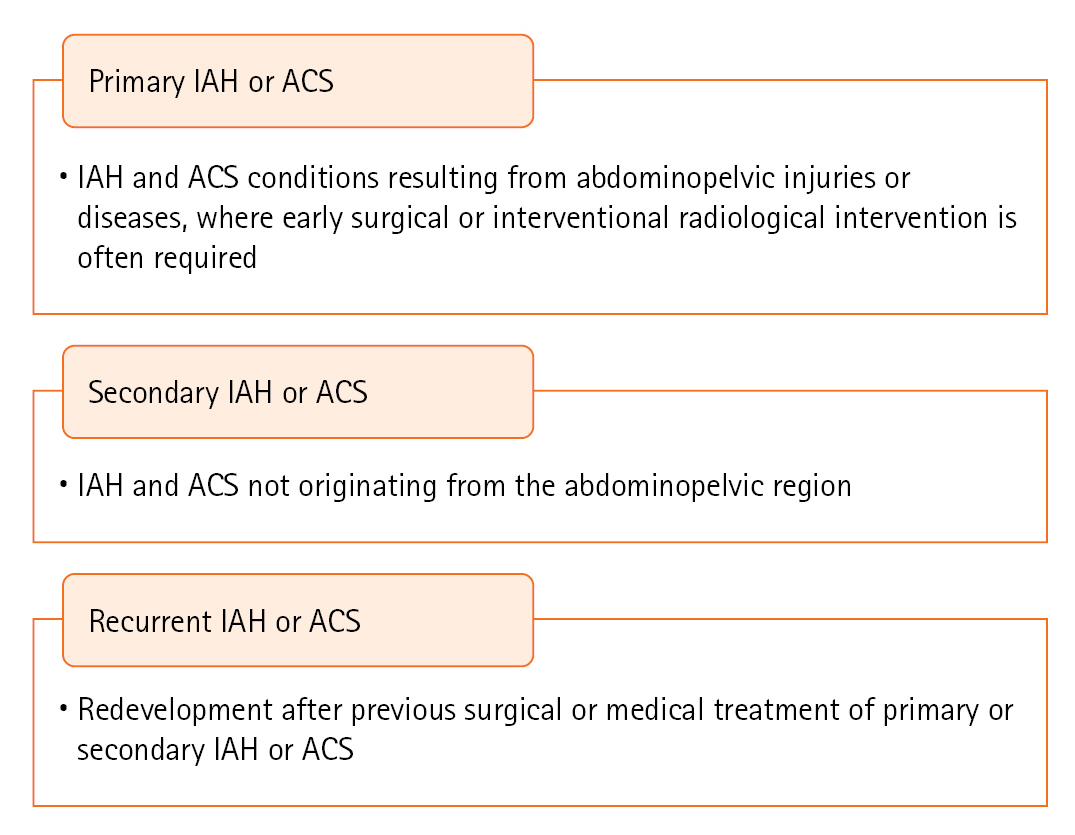
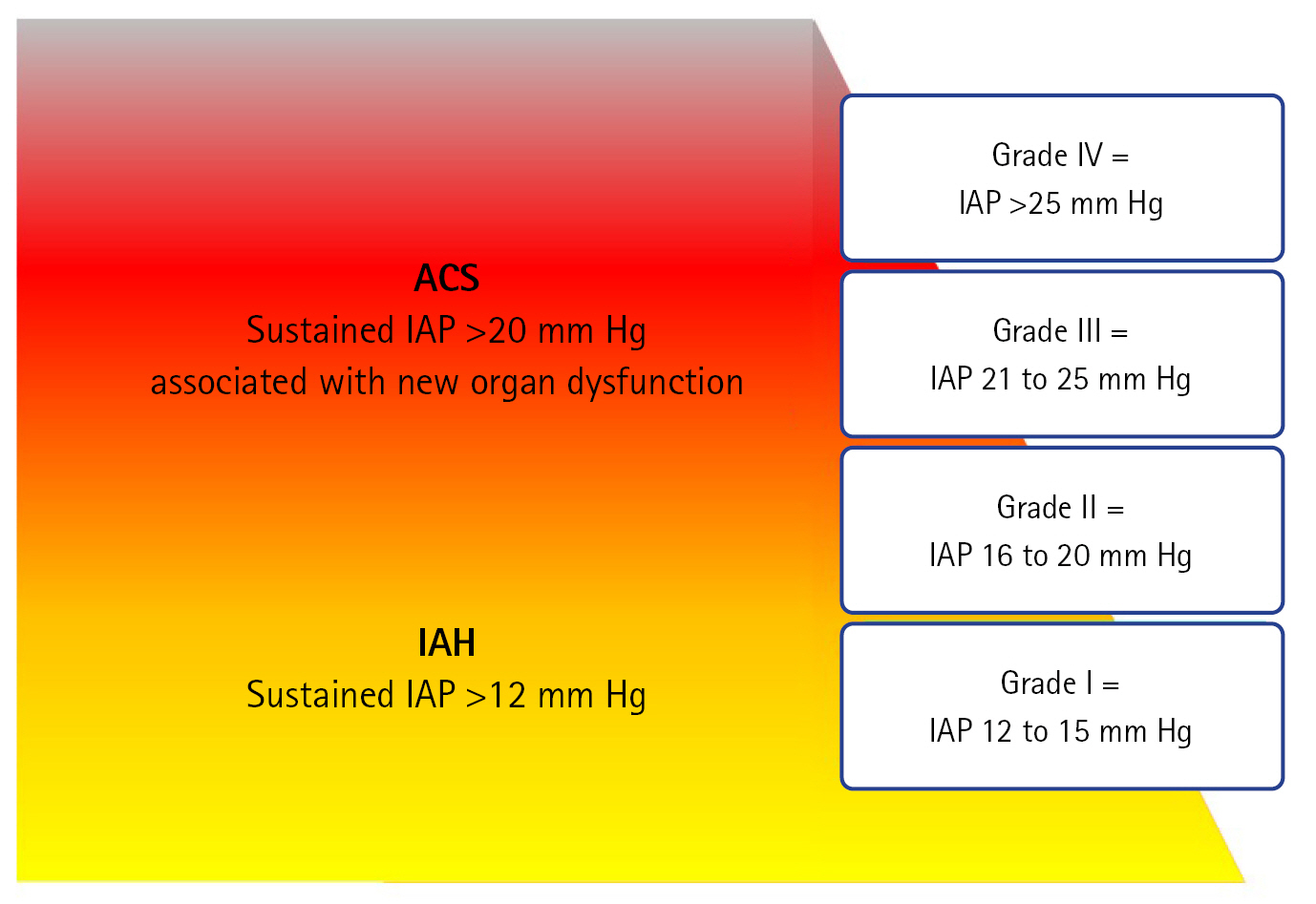
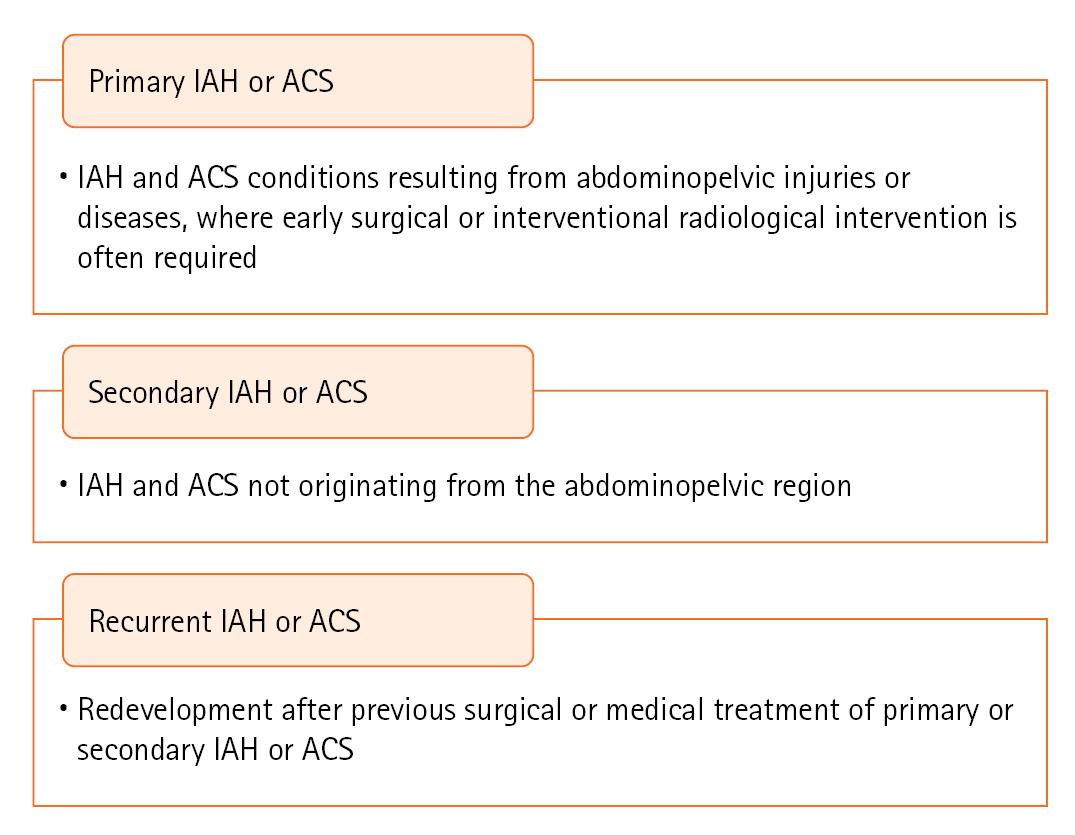
- PRIMARY IAH OR ACS DEFINITIONS
- IAH ASSESSMENT: INFLUENCE OF RISK FACTORS
- PROGRESSION OF IAH TO ACS AND ITS IMPACT ON VARIOUS ORGANS
- IMPACT ON THE KIDNEYS
- IMPACT ON THE RESPIRATORY SYSTEM
- IMPACT ON CIRCULATION
- IMPACT ON INTRACRANIAL PRESSURE
- IMPACT ON GASTROINTESTINAL TRACT AND HEPATOBILIARY SYSTEM
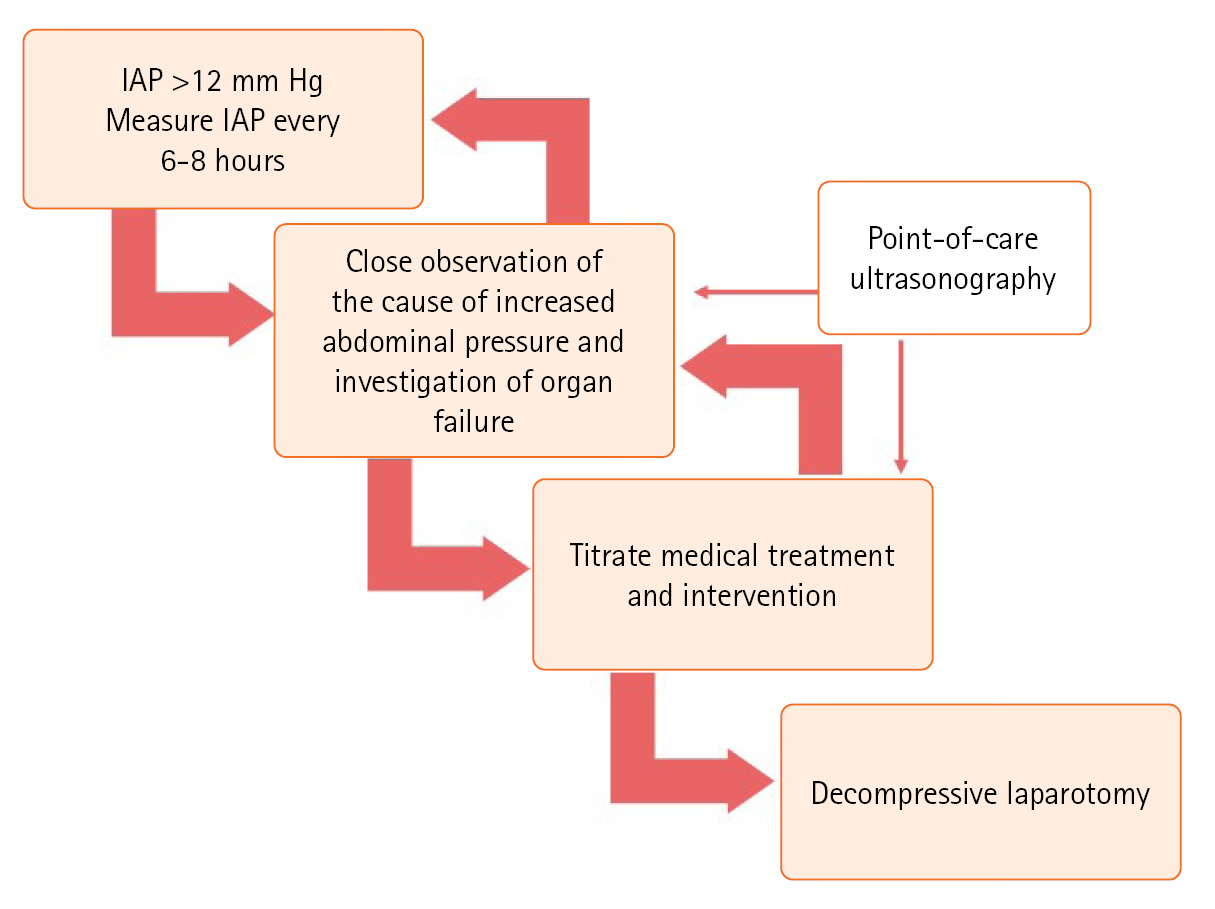
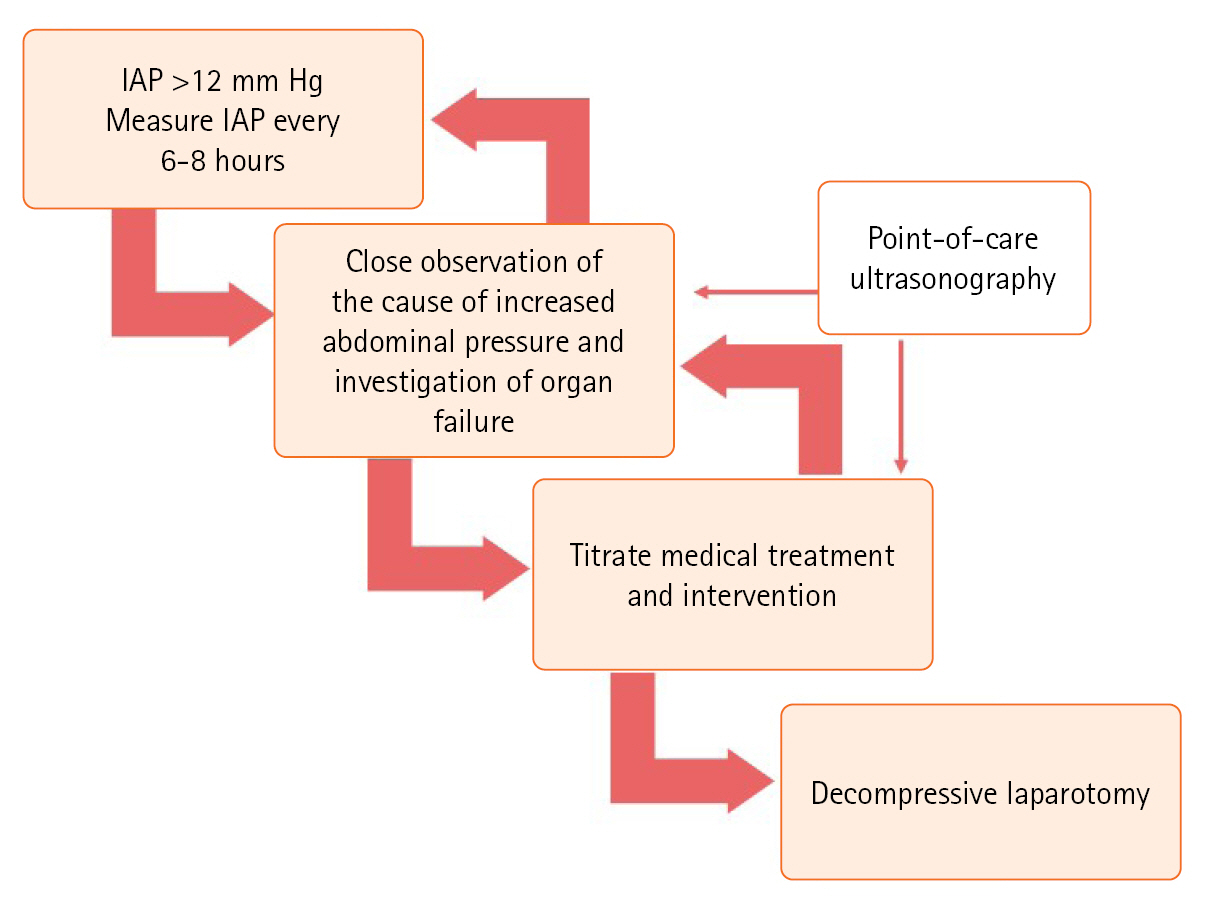
- TREATMENT AND MONITORING
- ELASTICITY OF ABDOMINAL WALL AND DIAPHRAGM (DIMINISHED ABDOMINAL WALL COMPLIANCE)
- INCREASED INTRALUMINAL AND ABDOMINAL CONTENT
- SYSTEMIC CONDITION DUE TO CAPILLARY LEAK OR FLUID RESUSCITATION
- DECOMPRESSIVE LAPAROTOMY
- CONCLUSIONS
- KEY MESSAGES
- NOTES
- Acknowledgments
- References
Abstract
- Intra-abdominal hypertension can have severe consequences, including abdominal compartment syndrome, which can contribute to multi-organ failure. An increase in intra-abdominal hypertension is influenced by factors such as diminished abdominal wall compliance, increased intraluminal content, and certain systemic conditions. Regular measurement of intra-abdominal pressure is essential, and particular attention must be paid to patient positioning. Nonsurgical treatments, such as decompression of intraluminal content using a nasogastric tube, percutaneous drainage, and fluid balance optimization, play crucial roles. Additionally, point-of-care ultrasonography aids in the diagnosis and treatment of intra-abdominal hypertension. Emphasizing the importance of regular measurements, timely decompressive laparotomy is a definitive, but complex, treatment option. Balancing the urgency of surgical intervention against potential postoperative complications is challenging.
INTRODUCTION
PATHOPHYSIOLOGY
DIAGNOSIS
MEASUREMENTS
PRIMARY IAH OR ACS DEFINITIONS
IAH ASSESSMENT: INFLUENCE OF RISK FACTORS
PROGRESSION OF IAH TO ACS AND ITS IMPACT ON VARIOUS ORGANS
IMPACT ON THE KIDNEYS
IMPACT ON THE RESPIRATORY SYSTEM
IMPACT ON CIRCULATION
IMPACT ON INTRACRANIAL PRESSURE
IMPACT ON GASTROINTESTINAL TRACT AND HEPATOBILIARY SYSTEM
TREATMENT AND MONITORING
ELASTICITY OF ABDOMINAL WALL AND DIAPHRAGM (DIMINISHED ABDOMINAL WALL COMPLIANCE)
INCREASED INTRALUMINAL AND ABDOMINAL CONTENT
SYSTEMIC CONDITION DUE TO CAPILLARY LEAK OR FLUID RESUSCITATION
DECOMPRESSIVE LAPAROTOMY
CONCLUSIONS
KEY MESSAGES
-
CONFLICT OF INTEREST
Dowan Kim is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: HJ,. Methodology: NL, JK. Formal analysis: EJ. Data curation: HJ, NL. Visualization: HJ, YP. Project administration: DK. Writing–original draft: HJ. Writing–review & editing: HJ, DK, YP, YJ.
NOTES
Acknowledgments
| Category | Risk factor | Reference |
|---|---|---|
| Elasticity of abdominal wall and diaphragm (diminished abdominal wall compliance) | Abdominal surgery | [14] |
| Major trauma | [15] | |
| Major burns | [16] | |
| Prone positioning | [17] | |
| High PEEP | [18] | |
| Increased intraluminal content | Gastroparesis/gastric distention | [19] |
| Ileus | [20]a) | |
| Colonic pseudo-obstruction | [21]a) | |
| Volvulus | [22]a) | |
| Increased abdominal content | Acute pancreatitis | [23] |
| Distended abdomen | [24] | |
| Hemoperitoneum/intra-peritoneal fluid collections | [25,26] | |
| Intra-abdominal infection/abscess | [27] | |
| Intra-abdominal or retroperitoneal mass | [28] | |
| Laparoscopy with excessive insufflation pressure | [29] | |
| Liver cirrhosis with ascites | [30] | |
| Peritoneal dialysis | [31] | |
| Systemic condition due to capillary leak/fluid resuscitation | Acidosis | [11] |
| Massive fluid resuscitation or massive transfusion | [32] | |
| Extracorporeal membrane oxygenation | [33] | |
| Sepsis | [34] | |
| Underlying cause | Head elevation state in bed | [35] |
| Obesity | [36] | |
| Peritonitis | [37] | |
| Pregnancy | [38] |
- 1. Malbrain ML, Chiumello D, Cesana BM, Reintam Blaser A, Starkopf J, Sugrue M, et al. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014;80:293-306.PubMed
- 2. Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 2005;33:315-22.ArticlePubMed
- 3. Reintam Blaser A, Regli A, De Keulenaer B, Kimball EJ, Starkopf L, Davis WA, et al. Incidence, risk factors, and outcomes of intra-abdominal hypertension in critically ill patients: a prospective multicenter study (IROI Study). Crit Care Med 2019;47:535-42.ArticlePubMedPMC
- 4. Malbrain ML, Deeren D, De Potter TJ. Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care 2005;11:156-71.ArticlePubMed
- 5. Vlot J, Wijnen R, Stolker RJ, Bax KN. Optimizing working space in laparoscopy: CT measurement of the effect of pre-stretching of the abdominal wall in a porcine model. Surg Endosc 2014;28:841-6.ArticlePubMedPDF
- 6. Malbrain ML, Peeters Y, Wise R. The neglected role of abdominal compliance in organ-organ interactions. Crit Care 2016;20:67. ArticlePubMedPMCPDF
- 7. Song C, Alijani A, Frank T, Hanna GB, Cuschieri A. Mechanical properties of the human abdominal wall measured in vivo during insufflation for laparoscopic surgery. Surg Endosc 2006;20:987-90.ArticlePubMedPDF
- 8. Petro CC, Raigani S, Fayezizadeh M, Rowbottom JR, Klick JC, Prabhu AS, et al. Permissible intraabdominal hypertension following complex abdominal wall reconstruction. Plast Reconstr Surg 2015;136:868-81.ArticlePubMed
- 9. Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg 2003;138:637-43.ArticlePubMed
- 10. Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015;43:68-73.ArticlePubMedPMC
- 11. Vidal MG, Ruiz Weisser J, Gonzalez F, Toro MA, Loudet C, Balasini C, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med 2008;36:1823-31.ArticlePubMed
- 12. Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190-206.ArticlePubMedPMCPDF
- 13. Kirkpatrick AW, Sugrue M, McKee JL, Pereira BM, Roberts DJ, De Waele JJ, et al. Update from the Abdominal Compartment Society (WSACS) on intra-abdominal hypertension and abdominal compartment syndrome: past, present, and future beyond Banff 2017. Anaesthesiol Intensive Ther 2017;49:83-7.ArticlePubMed
- 14. Ersryd S, Gidlund KD, Wanhainen A, Smith L, Björck M. Editor's choice: abdominal compartment syndrome after surgery for abdominal aortic aneurysm: subgroups, risk factors, and outcome. Eur J Vasc Endovasc Surg 2019;58:671-9.Article
- 15. He L, Yi C, Hou Z, Hak DJ. Intraabdominal hypertension/abdominal compartment syndrome after pelvic fractures: How they occur and what can be done? Injury 2019;50:919-25.ArticlePubMed
- 16. Struck MF, Reske AW, Schmidt T, Hilbert P, Steen M, Wrigge H. Respiratory functions of burn patients undergoing decompressive laparotomy due to secondary abdominal compartment syndrome. Burns 2014;40:120-6.ArticlePubMed
- 17. Yi M, Leng Y, Bai Y, Yao G, Zhu X. The evaluation of the effect of body positioning on intra-abdominal pressure measurement and the effect of intra-abdominal pressure at different body positioning on organ function and prognosis in critically ill patients. J Crit Care 2012;27:222. Article
- 18. Regli A, Pelosi P, Malbrain MLNG. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care 2019;9:52. ArticlePubMedPMCPDF
- 19. Parkman HP, Hasler WL, Fisher RS, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004;127:1592-622.ArticlePubMed
- 20. Madl C, Druml W. Gastrointestinal disorders of the critically ill. Systemic consequences of ileus. Best Pract Res Clin Gastroenterol 2003;17:445-56.PubMed
- 21. Granero Castro P, Fernández Arias S, Moreno Gijón M, Alvarez Martínez P, Granero Trancón J, Álvarez Pérez JA, et al. Emergency surgery in chronic intestinal pseudo-obstruction due to mitochondrial neurogastrointestinal encephalomyopathy: case reports. Int Arch Med 2010;3:35. ArticlePubMedPMC
- 22. Marin AC, Hechter S, Prasad A, Alnabwani D, Lwoodsky C, Cheriyath P. Abdominal compartment syndrome (ACS) with sigmoid volvulus (SV): lost hours are lost lives. Cureus 2023;15:e33741.ArticlePubMedPMC
- 23. Marcos-Neira P, Zubia-Olaskoaga F, López-Cuenca S, Bordejé-Laguna L, Epidemiology of Acute Pancreatitis in Intensive Care Medicine study group. Relationship between intra-abdominal hypertension, outcome and the revised Atlanta and determinant-based classifications in acute pancreatitis. BJS Open 2018;1:175-81.ArticlePubMedPMCPDF
- 24. Bouveresse S, Piton G, Badet N, Besch G, Pili-Floury S, Delabrousse E. Abdominal compartment syndrome and intra-abdominal hypertension in critically ill patients: diagnostic value of computed tomography. Eur Radiol 2019;29:3839-46.ArticlePubMedPDF
- 25. Singh AK, Samanta J, Dawra S, Gupta P, Rana A, Sharma V, et al. Reduction of intra-abdominal pressure after percutaneous catheter drainage of pancreatic fluid collection predicts survival. Pancreatology 2020;20:772-7.ArticlePubMed
- 26. Battey TW, Dreizin D, Bodanapally UK, Wnorowski A, Issa G, Iacco A, et al. A comparison of segmented abdominopelvic fluid volumes with conventional CT signs of abdominal compartment syndrome in a trauma population. Abdom Radiol (NY) 2019;44:2648-55.ArticlePubMedPDF
- 27. Murphy PB, Parry NG, Sela N, Leslie K, Vogt K, Ball I. Intra-abdominal hypertension is more common than previously thought: a prospective study in a mixed medical-surgical ICU. Crit Care Med 2018;46:958-64.ArticlePubMed
- 28. Daliakopoulos SI, Schaedel M, Klimatsidas MN, Spiliopoulos S, Koerfer R, Tenderich G. Intra-abdominal hypertension due to heparin-induced retroperitoneal hematoma in patients with ventricle assist devices: report of four cases and review of the literature. J Cardiothorac Surg 2010;5:108. ArticlePubMedPMCPDF
- 29. Raval AD, Deshpande S, Koufopoulou M, Rabar S, Neupane B, Iheanacho I, et al. The impact of intra-abdominal pressure on perioperative outcomes in laparoscopic cholecystectomy: a systematic review and network meta-analysis of randomized controlled trials. Surg Endosc 2020;34:2878-90.ArticlePubMedPMCPDF
- 30. Pereira R, Buglevski M, Perdigoto R, Marcelino P, Saliba F, Blot S, et al. Intra-abdominal hypertension and abdominal compartment syndrome in the critically ill liver cirrhotic patient-prevalence and clinical outcomes: a multicentric retrospective cohort study in intensive care. PLoS One 2021;16:e0251498.ArticlePubMedPMC
- 31. Castellanos LB, Clemente EP, Cabañas CB, Parra DM, Contador MB, Morera JC, et al. Clinical relevance of intraperitoneal pressure in peritoneal dialysis patients. Perit Dial Int 2017;37:562-7.ArticlePubMedPDF
- 32. Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med 2008;34:707-13.ArticlePubMedPDF
- 33. Glowka TR, Schewe JC, Muenster S, Putensen C, Kalff JC, Pantelis D. Decompressive laparotomy for the treatment of the abdominal compartment syndrome during extracorporeal membrane oxygenation support. J Crit Care 2018;47:274-9.ArticlePubMed
- 34. Pereira B, Dorigatti A, Melek M, Dos Santos J, Ferreira M, Calderan T, et al. Septic shock patients admitted to the intensive care unit with higher SOFA score tend to have higher incidence of abdominal compartment syndrome: a preliminary analysis. Anaesthesiol Intensive Ther 2019;51:370-2.ArticlePubMed
- 35. Cheatham ML, De Waele JJ, De Laet I, De Keulenaer B, Widder S, Kirkpatrick AW, et al. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009;37:2187-90.ArticlePubMed
- 36. Pereira BM, Pereira RG, Wise R, Sugrue G, Zakrison TL, Dorigatti AE, et al. The role of point-of-care ultrasound in intra-abdominal hypertension management. Anaesthesiol Intensive Ther 2017;49:373-81.ArticlePubMed
- 37. Basu A, Pai DR. Early elevation of intra-abdominal pressure after laparotomy for secondary peritonitis: a predictor of relaparotomy? World J Surg 2008;32:1851-6.ArticlePubMedPDF
- 38. Chun R, Kirkpatrick AW. Intra-abdominal pressure, intra-abdominal hypertension, and pregnancy: a review. Ann Intensive Care 2012;2 Suppl 1:S5. PubMed
- 39. Kyoung KH, Hong SK. The duration of intra-abdominal hypertension strongly predicts outcomes for the critically ill surgical patients: a prospective observational study. World J Emerg Surg 2015;10:22. ArticlePubMedPMCPDF
- 40. De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis 2011;57:159-69.ArticlePubMed
- 41. Smit M, Hofker HS, Leuvenink HG, Krikke C, Jongman RM, Zijlstra JG, et al. A human model of intra-abdominal hypertension: even slightly elevated pressures lead to increased acute systemic inflammation and signs of acute kidney injury. Crit Care 2013;17:425. ArticlePubMedPMC
- 42. Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol 2011;22:615-21.ArticlePubMed
- 43. Papakrivou E, Makris D, Manoulakas E, Karvouniaris M, Zakynthinos E. Intra-abdominal hypertension is a risk factor for increased VAP incidence: a prospective cohort study in the ICU of a tertiary hospital. J Intensive Care Med 2020;35:700-7.ArticlePubMedPDF
- 44. Malbrain ML, De Waele JJ, De Keulenaer BL. What every ICU clinician needs to know about the cardiovascular effects caused by abdominal hypertension. Anaesthesiol Intensive Ther 2015;47:388-99.ArticlePubMed
- 45. Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma 1995;39:1071-5.ArticlePubMed
- 46. Citerio G, Vascotto E, Villa F, Celotti S, Pesenti A. Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: a prospective study. Crit Care Med 2001;29:1466-71.ArticlePubMed
- 47. Montorfano L, Giambartolomei G, Funes DR, Lo Menzo E, Dip F, White KP, et al. The Cushing reflex and the vasopressin-mediated hemodynamic response to increased intracranial pressure during acute elevations in intraabdominal pressure. Surgery 2020;167:478-83.ArticlePubMed
- 48. Diebel LN, Dulchavsky SA, Brown WJ. Splanchnic ischemia and bacterial translocation in the abdominal compartment syndrome. J Trauma 1997;43:852-5.ArticlePubMed
- 49. Khripun AI, Ettinger AP, Chadaev AP, Tat'kov SS. Changes in the contractile and bioelectrical activities of the rat small intestine under increased intra-abdominal pressure. Izv Akad Nauk Ser Biol 1997;596-602.PubMed
- 50. Mogilner JG, Bitterman H, Hayari L, Brod V, Coran AG, Shaoul R, et al. Effect of elevated intra-abdominal pressure and hyperoxia on portal vein blood flow, hepatocyte proliferation and apoptosis in a rat model. Eur J Pediatr Surg 2008;18:380-6.ArticlePubMed
- 51. Antoniou EA, Kairi E, Margonis GA, Andreatos N, Sasaki K, Damaskos C, et al. Effect of increased intra-abdominal pressure on liver histology and hemodynamics: an experimental study. In Vivo 2018;32:85-91.PubMedPMC
- 52. Malbrain ML, Roberts DJ, Sugrue M, De Keulenaer BL, Ivatury R, Pelosi P, et al. The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther 2014;46:433-50.ArticlePubMed
- 53. De Laet I, Hoste E, Verholen E, De Waele JJ. The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med 2007;33:1811-4.ArticlePubMedPDF
- 54. Oda J, Ueyama M, Yamashita K, Inoue T, Harunari N, Ode Y, et al. Effects of escharotomy as abdominal decompression on cardiopulmonary function and visceral perfusion in abdominal compartment syndrome with burn patients. J Trauma 2005;59:369-74.ArticlePubMed
- 55. Butts CC, Holmes JH, Carter JE. Surgical escharotomy and decompressive therapies in burns. J Burn Care Res 2020;41:263-9.ArticlePubMedPDF
- 56. Parra MW, Al-Khayat H, Smith HG, Cheatham ML. Paracentesis for resuscitation-induced abdominal compartment syndrome: an alternative to decompressive laparotomy in the burn patient. J Trauma 2006;60:1119-21.ArticlePubMed
- 57. Xu JM, Yang HD, Tian XP. Effects of early hemofiltration on organ function and intra-abdominal pressure in severe acute pancreatitis patients with abdominal compartment syndrome. Clin Nephrol 2019;92:243-9.ArticlePubMed
- 58. Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med 2010;38:1824-9.ArticlePubMed
- 59. Beurton A, Teboul JL, Girotto V, Galarza L, Anguel N, Richard C, et al. Intra-abdominal hypertension is responsible for false negatives to the passive leg raising test. Crit Care Med 2019;47:e639-47.ArticlePubMed
- 60. García AF, Sánchez ÁI, Gutiérrez ÁJ, Bayona JG, Naranjo MP, Lago S, et al. Effect of abdominal negative-pressure wound therapy on the measurement of intra-abdominal pressure. J Surg Res 2018;227:112-8.ArticlePubMed
- 61. Patel NY, Cogbill TH, Kallies KJ, Mathiason MA. Temporary abdominal closure: long-term outcomes. J Trauma 2011;70:769-74.ArticlePubMed
References
Figure & Data
References
Citations

- Figure
- We recommend
- Related articles
-
- Brain–computer interface in critical care and rehabilitation
- The effects of environmental interventions for delirium in critically ill surgical patients
- The incidence of acute acalculous cholecystitis in critically ill COVID-19 patients
- Clinical characteristics and outcomes of critically ill COVID-19 patients in Sfax, Tunisia

 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite