Articles
- Page Path
- HOME > Acute Crit Care > Volume 39(1); 2024 > Article
-
Original Article
Trauma The correlation between carotid artery Doppler and stroke volume during central blood volume loss and resuscitation -
Isabel Kerrebijn1
 , Sarah Atwi1
, Sarah Atwi1 , Mai Elfarnawany1
, Mai Elfarnawany1 , Andrew M. Eibl1,2, Joseph K. Eibl1,2,3
, Andrew M. Eibl1,2, Joseph K. Eibl1,2,3 , Jenna L. Taylor4,5
, Jenna L. Taylor4,5 , Chul-Ho Kim4, Bruce D. Johnson4, Jon-Émile S. Kenny1,2
, Chul-Ho Kim4, Bruce D. Johnson4, Jon-Émile S. Kenny1,2
-
Acute and Critical Care 2024;39(1):162-168.
DOI: https://doi.org/10.4266/acc.2023.01095
Published online: February 23, 2024
1Flosonics Medical, Toronto, ON, Canada
2Health Sciences North Research Institute, Sudbury, ON, Canada
3Northern Ontario School of Medicine University, Sudbury, ON, Canada
4Human Integrative and Environmental Physiology Laboratory, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
5Physiology and Ultrasound Laboratory in Science and Exercise, School of Human Movement and Nutrition Sciences, The University of Queensland, Brisbane, Australia
- Corresponding author: Jon-Émile S. Kenny Flosonics Medical, 325 W. Front St, Toronto, ON M5V2Y1, Canada Tel: +1-705-523-7100 Email: jon-emile@heart-lung.org
© 2024 The Korean Society of Critical Care Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 496 Views
- 38 Download
Abstract
-
Background
- Using peripheral arteries to infer central hemodynamics is common among hemodynamic monitors. Doppler ultrasound of the common carotid artery has been used in this manner with conflicting results. We investigated the relationship between changing common carotid artery Doppler measures and stroke volume (SV), hypothesizing that more consecutively-averaged cardiac cycles would improve SV-carotid Doppler correlation.
-
Methods
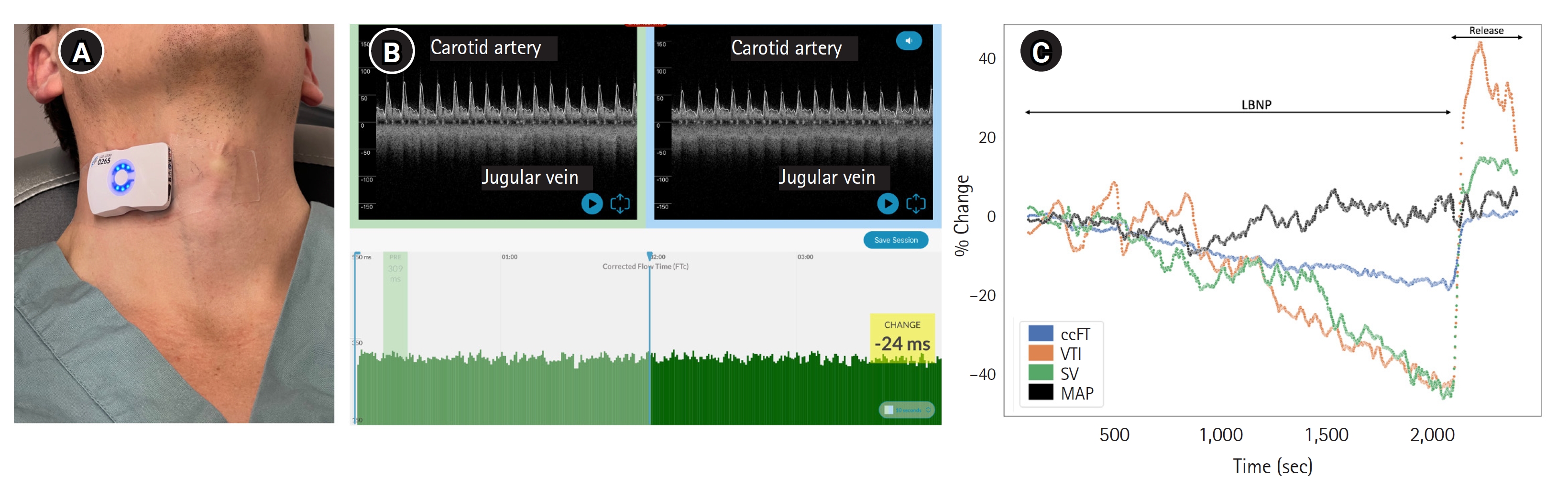
- Twenty-seven healthy volunteers were recruited and studied in a physiology laboratory. Carotid artery Doppler pulse was measured with a wearable, wireless ultrasound during central hypovolemia and resuscitation induced by a stepped lower body negative pressure protocol. The change in maximum velocity time integral (VTI) and corrected flow time of the carotid artery (ccFT) were compared with changing SV using repeated measures correlation.
-
Results
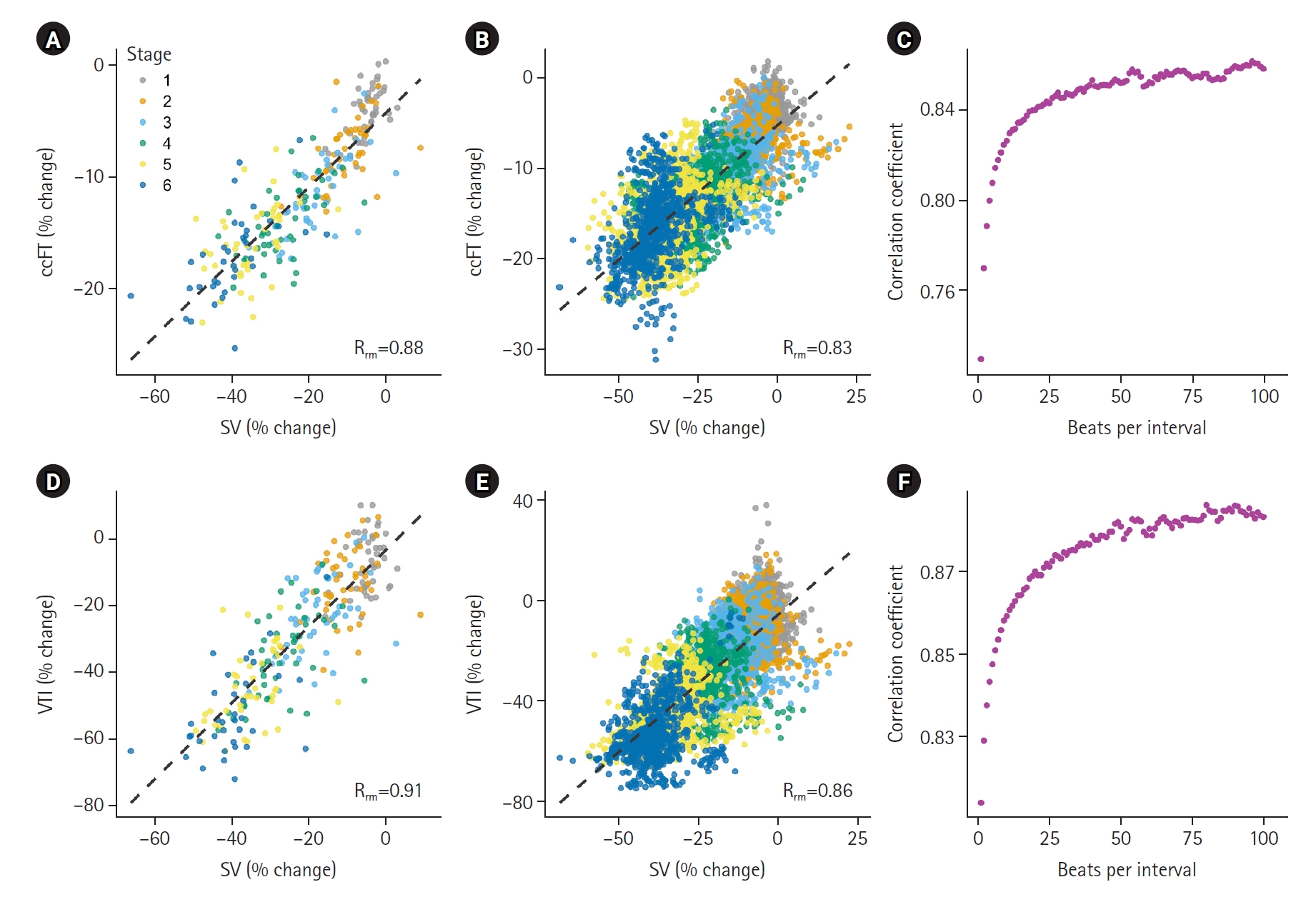
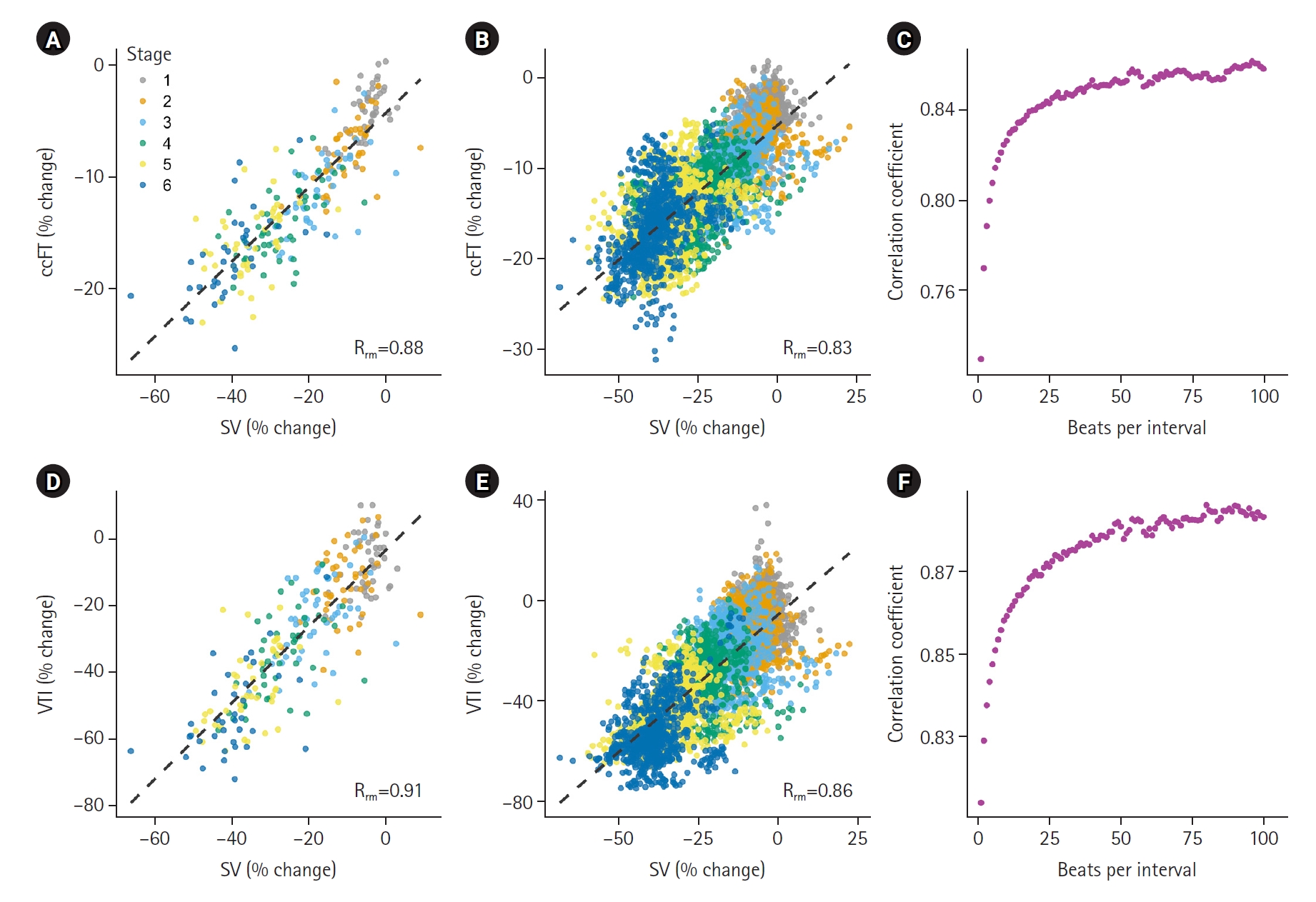
- In total, 73,431 cardiac cycles were compared across 27 subjects. There was a strong linear correlation between changing SV and carotid Doppler measures during simulated hemorrhage (repeated-measures linear correlation [Rrm]=0.91 for VTI; 0.88 for ccFT). This relationship improved with larger numbers of consecutively-averaged cardiac cycles. For ccFT, beyond four consecutively-averaged cardiac cycles the correlation coefficient remained strong (i.e., Rrm of at least 0.80). For VTI, the correlation coefficient with SV was strong for any number of averaged cardiac cycles. For both ccFT and VTI, Rrm remained stable around 25 consecutively-averaged cardiac cycles.
-
Conclusions
- There was a strong linear correlation between changing SV and carotid Doppler measures during central blood volume loss. The strength of this relationship was dependent upon the number of consecutively-averaged cardiac cycles.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
HIGHLIGHTS
-
CONFLICT OF INTEREST
IK, SA, ME, AME, JKE, JESK are employees of Flosonics Medical. No other potential conflicts of interest relevant to this article were reported.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: ME, AE, JKE, JLT, CHK, BDJ, JESK. Methodology: ME, AE, JKE, JLT, CHK, BDJ, JESK. Formal analysis: IK, SA. Data curation: IK, JLT. Visualization: IK, SA. Project administration: ME, AE, JKE, JESK. Funding acquisition: AE, JKE, JESK. Writing–original draft: IK, JESK. Writing–review & editing: all authors. All authors read and agreed to the published version of the manuscript.
-
ACKNOWLEDGMENTS
None.
NOTES
- 1. Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016;42:1350-9.ArticlePubMedPDF
- 2. Cheong I, Otero Castro V, Sosa FA, Tort Oribe B, Merlo PM, Tamagnone FM. Carotid flow as a surrogate of the left ventricular stroke volume. J Clin Monit Comput 2023;37:661-7.ArticlePubMedPDF
- 3. Kenny JS. A theoretical foundation for relating the velocity time integrals of the left ventricular outflow tract and common carotid artery. J Clin Monit Comput 2023;37:937-9.ArticlePubMedPMCPDF
- 4. Kenny JS, Barjaktarevic I, Mackenzie DC, Elfarnawany M, Yang Z, Eibl AM, et al. Carotid Doppler ultrasonography correlates with stroke volume in a human model of hypovolaemia and resuscitation: analysis of 48 570 cardiac cycles. Br J Anaesth 2021;127:e60-3.ArticlePubMed
- 5. Kenny JS, Barjaktarevic I, Eibl AM, Parrotta M, Long BF, Elfarnawany M, et al. Temporal concordance between pulse contour analysis, bioreactance and carotid doppler during rapid preload changes. PLoS One 2022;17:e0265711. ArticlePubMedPMC
- 6. Barjaktarevic I, Toppen WE, Hu S, Aquije Montoya E, Ong S, Buhr R, et al. Ultrasound assessment of the change in carotid corrected flow time in fluid responsiveness in undifferentiated shock. Crit Care Med 2018;46:e1040-6.ArticlePubMedPMC
- 7. Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest 2013;143:364-70.ArticlePubMed
- 8. Effat H, Hamed K, Hamed G, Mostafa R, El Hadidy S. Electrical cardiometry versus carotid Doppler in assessment of fluid responsiveness in critically ill septic patients. Egypt J Crit Care Med 2021;8:96-113.
- 9. Kimura A, Suehiro K, Juri T, Tanaka K, Mori T. Changes in corrected carotid flow time induced by recruitment maneuver predict fluid responsiveness in patients undergoing general anesthesia. J Clin Monit Comput 2022;36:1069-77.ArticlePubMedPDF
- 10. Jung S, Kim J, Na S, Nam WS, Kim DH. Ability of carotid corrected flow time to predict fluid responsiveness in patients mechanically ventilated using low tidal volume after surgery. J Clin Med 2021;10:2676. ArticlePubMedPMC
- 11. Cheong I, Otero Castro V, Sosa FA, Tort Oribe B, Fruchtenicht MF, Tamagnone FM, et al. Passive leg raising test using the carotid flow velocity-time integral to predict fluid responsiveness. J Ultrasound 2023 Sep 5 [Epub];https://doi.org/10.1007/s40477-023-00824-1.Article
- 12. Douglas IS, Alapat PM, Corl KA, Exline MC, Forni LG, Holder AL, et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest 2020;158:1431-45.ArticlePubMedPMC
- 13. Dubin A, Loudet C, Kanoore Edul VS, Osatnik J, Ríos F, Vásquez D, et al. Characteristics of resuscitation, and association between use of dynamic tests of fluid responsiveness and outcomes in septic patients: results of a multicenter prospective cohort study in Argentina. Ann Intensive Care 2020;10:40. ArticlePubMedPMCPDF
- 14. Kenny JS, Elfarnawany M, Yang Z, Eibl AM, Eibl JK, Kim CH, et al. A wireless ultrasound patch detects mild-to-moderate central hypovolemia during lower body negative pressure. J Trauma Acute Care Surg 2022;93(2S Suppl 1):S35-40.ArticlePubMedPMC
- 15. Abbasi A, Azab N, Nayeemuddin M, Schick A, Lopardo T, Phillips GS, et al. Change in carotid blood flow and carotid corrected flow time assessed by novice sonologists fails to determine fluid responsiveness in spontaneously breathing intensive care unit patients. Ultrasound Med Biol 2020;46:2659-66.ArticlePubMedPMC
- 16. van Houte J, Mooi FJ, Montenij LJ, Meijs LP, Suriani I, Conjaerts BC, et al. Correlation of carotid Doppler blood flow with invasive cardiac output measurements in cardiac surgery patients. J Cardiothorac Vasc Anesth 2022;36:1081-91.ArticlePubMed
- 17. Khan S, Mishra SK, Parida S, Jha AK, Nagabhushanam Padala SR. Carotid doppler indices do not predict fluid responsiveness in mechanically ventilated patients undergoing coronary artery bypass grafting surgery. J Card Surg 2022;37:4418-24.ArticlePubMedPDF
- 18. Kenny JS, Barjaktarevic I, Mackenzie DC, Elfarnawany M, Math ZY, Eibl AM, et al. Carotid Doppler measurement variability in functional hemodynamic monitoring: an analysis of 17,822 cardiac cycles. Crit Care Explor 2021;3:e0439. ArticlePubMedPMC
- 19. Bussmann BM, Sharma S, Mcgregor D, Hulme W, Harris T. Observational study in healthy volunteers to define interobserver reliability of ultrasound haemodynamic monitoring techniques performed by trainee doctors. Eur J Emerg Med 2019;26:217-23.ArticlePubMed
- 20. Kenny JS, Barjaktarevic I, Mackenzie DC, Elfarnawany M, Yang Z, Eibl AM, et al. Carotid artery velocity time integral and corrected flow time measured by a wearable Doppler ultrasound detect stroke volume rise from simulated hemorrhage to transfusion. BMC Res Notes 2022;15:7. ArticlePubMedPMCPDF
- 21. Kerrebijn I, Atwi S, Horner C, Elfarnawany M, Eibl AM, Eibl JK, et al. Correlation between changing carotid artery corrected flow time and ascending aortic Doppler flow velocity. Br J Anaesth 2023;131:e192-5.ArticlePubMed
- 22. Kenny JS, Elfarnawany M, Yang Z, Myers M, Eibl AM, Eibl JK, et al. The Doppler shock index measured by a wearable ultrasound patch accurately detects moderate-to-severe central hypovolemia during lower body negative pressure. J Am Coll Emerg Physicians Open 2021;2:e12533. ArticlePubMedPMCPDF
- 23. Kenny JS, Cannesson M, Barjaktarevic I. Minimizing measurement variability in carotid ultrasound evaluations. J Ultrasound Med 2021;40:855-6.ArticlePubMedPMCPDF
- 24. Kenny JS, Barjaktarevic I. Timing and measurement variability are critical when using carotid Doppler to infer hemodynamics. Ultrasound Med Biol 2020;46:3485-6.ArticlePubMed
- 25. Beier L, Davis J, Esener D, Grant C, Fields JM. Carotid ultrasound to predict fluid responsiveness: a systematic review. J Ultrasound Med 2020;39:1965-76.PubMed
References
Figure & Data
References
Citations


 KSCCM
KSCCM
 PubReader
PubReader ePub Link
ePub Link Cite
Cite